|
 |
 |
Dr. Akihiko Takashima
Head, Laboratory for Alzheimer's Disease |
 |

|
Alzheimer's Disease
|
 |
Introduction |
|
It was in 1906 that Dr. Alzheimer reported on the clinical history and pathological observations of a woman aged 51 years who suffered from amnesia.This was the first report of what is presently called Alzheimer's disease (AD).
|
|
Today, the name of Alzheimer's disease has become a generic name which includes senile dementia syndromes presenting similar pathological symptoms This disease initiates from the degradation of memory capability, that is, the impossibility of recalling what the patient has memorized before. In the metaphase, the disease condition progresses to the loss of cognition capability. In this stage, the patient cannot recall the current date or the place where he or she is now, and severe dementia eventually results. The disease progresses gradually over 10 to 20 years after the sideration until the death of the patient. Since its symptoms usually show in senescence, it is known as a representative senile dementia together with vascular dementia, and the number of patients in Japan is estimated to be about 600,000. For the present, regretfully, unlike the cases of vascular dementia we have no effective prophylaxis or therapy against AD.
Our research began by questioning why AD causes dementia and we ultimately aim through our research at identifying the molecular mechanisms causing the dementia. The dementia refers to the state in which the intellectual and spiritual capabilities acquired by an individual are lost and unrecoverable. A person must first acquire intellectual and spiritual capabilities in order to fall into dementia. However, the process of acquisition of intellectual and spiritual capabilities itself poses a big problem if we attempt to explain it by using molecular terminology. This is because the molecular mechanism of acquiring these capabilities itself has not yet been clarified and remains one of the major issues of brain science. In consequence, the elucidation of the pathology of AD not only provides the effective means of its prevention and treatment but also a clue for solving major issues of brain science.
|
 |
 |
Pathological Characteristics of Alzheimer's Disease |
 |
|
One of the difficulties in research into Alzheimer's disease lies in the fact that it is specific to human beings and not observed with other animals. As this fact limits the test subject to human brains, the research was commenced by investigating the pathological characteristics of Alzheimer's disease, that is, examining the differences between brains affected by Alzheimer's disease from normal human brains.
The first characteristic which is visible from the outside is the atrophy of the brain. This atrophy is caused by the loss of neurons. As the degree of dementia and that of atrophy are closely correlated with each other, it is regarded that dementia in Alzheimer's disease is a result of the loss of neurons. The second characteristic is the appearance of an insoluble aggregate called senile plaque.
|
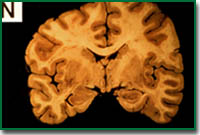
Normal
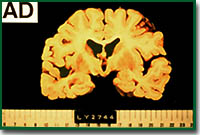
Alzheimer's disease
|
|
This plaque is observed in the brain just like spots on the skin. However, in reality, it can only be observed by staining a slice of the brain. Senile plaque is also observed in normal persons from an age of around 40 years and the amount increases with the age of the person.
In the case of AD brain, the number of senile plaques is much larger than in normal brain, are extended throughout the entire brain and are observed in locations where they are not observed in normal brains. From the viewpoint of ingredients, senile plaque is formed by a peptide composed of about 39 to 43 amino acids, called amyloid beta protein (Ab).
This peptide forms a part of the amyloid precursor protein (APP) which is a membrane protein, and is a degradation product of this protein.
In other words, senile plaque is the result of the aggregation and accumulation of Ab produced by alternative processing of APP and is secreted to extra cellular space. A third pathological characteristic is observed inside the neurons. An AD brain presents neurons containing fibrillar structures called neurofibrillary tangles (NFTs). Electron microscopy of these structures shows that they consist of paired helical filaments (PHFs)combining 10 nm diameter single filaments entangled at an interval of 80 nm.
|
|
The PHFs are composed of abnormally phosphorylated, tau proteins. The tau proteins are microtubule-binding proteins, which contribute to the stabilization of the cytoskeleton by binding with the microtubules when the tau proteins are highly phosphorylated, they loose the ability of binding to microtubules, resulting accumulation and aggregation of phosphorylated tau in neurons. It is considered as mechanisms of NFTs formation (Fig. 1) .
This neurofibrillary degeneration is also observed with diseases accompanied by dementia other than Alzheimer's disease, such as Pick's disease.
|
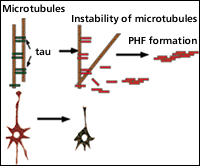
Fig.1 Abnormal Phosphorylation by GSK-3b
(magnify:36k)
|
As it has been found that the degree of dementia is proportional to the frequency of observation of neurons bearing NFTs, we believe that identifying the exact biochemical mechanism of NFTs formation will lead to the elucidation of the dementia.
|
 |
 |
Mechanism of Neurondeath in Alzheimer's Disease |
 |
|
Alzheimer's disease begins with an initial accumulation of senile plaque, which is followed by NFTs formation and loss of neurons a few decades later. And these pathological characteristics show the key to the clarification of what is happening in the Alzheimer's disease brain. One of the hypotheses describing this disease is the amyloid hypothesis (Fig. 2) , which postulates that it is due to the Ab protein. When Ab is secreted from cells, it accumulates outside the cells and become senile plaque. This is based on an idea that Ab directly affects the neurons, leading phosphorylation of tau proteins and results NFTs and neuronal death . Now, in this stage, it is no longer necessary to limit the subject of research into Alzheimer's diseases to human brains. All we have to do is investigate the mechanisms of neuron death and tau phosphorylation by amyloid.
Up to the present, by adding synthetic Ab to the primary cultured hippocampal neurons of rat fetuses we have succeeded in demonstrating that amyloid causes neuron death and tau phosphorylation, and have discovered that one of the enzymes activated in the neurons is glycogen synthase kinase (GSK-3b). This enzyme is capable of excessively phosphorylating the tau protein.
|
|
In addition to the tau, GSK-3b also phosphorylates the pyruvate dehydrogenase (PDH) which is a protein in the mitochondria and phosphorylation inhibits its activity. The inactivation of this enzyme reduces the production of acetyl co-A, which is a metabolic product, and leads to the reduction of ATP supply and eventually to their deaths. As the acetyl co-A is required for the synthesis of acetylcholine which is the transmitter in cholinergic neurons, the inactivation also leads to the impossibility of the synthesis of acetylcholine. This means an insufficiency in the neural transmission by not releasing enough acetylcholine against stimuli. Neurons containing acetylcholine are present in many locations of the brain and are involved in memory and learning. And the reduction of acetylcholine may explain the loss of memory observed in the initial stages of Alzheimer's disease.
|
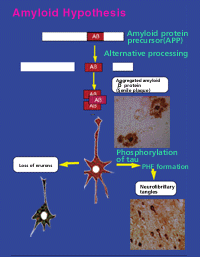
Fig.2 Amyloid Hypothesis
(magnify:63k)
|
|
As described above, we believe that we have succeeded in explaining the phenomena caused by Alzheimer's disease based on the activation of GSK-3b by amyloid. At present, we are attempting to demonstrate this hypothesis with individual animals by creating transgenic animals and inducing Alzheimer's disease in them.
|
 |
|
 |
 |
|
|