|
 |
 |
Zinc
suppresses the formation of fibrils of b amyloid, a causative protein of Alzheimerユs
disease
Laboratory for Alzheimer's
Disease |
|
 |
In
a brain affected with Alzheimerユs disease (AD), abnormal accumulation of particular
proteins is observed, and it is said that this accumulation would result in the
degeneration of nerve cells that are associated with memory in humans. One of
the proteins that accumulates abnormally in the brain is  amyloid
(A amyloid
(A ), which aggregates into amyloid
fibrils to form senile plaques. It is known that amyloid fibrils, which are formed
by ), which aggregates into amyloid
fibrils to form senile plaques. It is known that amyloid fibrils, which are formed
by  -aggregation of A -aggregation of A ,
are cytotoxic. We searched for substances that suppress the b-aggregation of Ab,
and we focused particularly on metal ions. ,
are cytotoxic. We searched for substances that suppress the b-aggregation of Ab,
and we focused particularly on metal ions.
Among metal ions, aluminum is most closely associated with AD. We verified how
various kinds of trace metal ions including aluminum influence the  -aggregation
of A -aggregation
of A . We found that zinc at a physiological
concentration suppresses the . We found that zinc at a physiological
concentration suppresses the  -aggregation
of A -aggregation
of A most effectively. On the other
hand, aluminum, at least by itself, did not markedly suppress the most effectively. On the other
hand, aluminum, at least by itself, did not markedly suppress the  -aggregation
of A -aggregation
of A . .
It is known from epidemiological studies that insufficient ingestion of zinc correlates
with an increase in the number of senile plaques. On the other hand, biochemical
studies have reported that metals such as zinc and copper accelerate the aggregation
of A . These two concepts are contradictory
in terms of aggregation of A . These two concepts are contradictory
in terms of aggregation of A induced
by zinc and the zinc effect on senile plaque formation. In this study, we reexamined
the effect of zinc using an assay method that detects specifically induced
by zinc and the zinc effect on senile plaque formation. In this study, we reexamined
the effect of zinc using an assay method that detects specifically  -aggregation
of A -aggregation
of A . This method utilizes a fluorescent
pigment, thioflavine(ThT), whose wavelength shifts when it binds to proteins with
a . This method utilizes a fluorescent
pigment, thioflavine(ThT), whose wavelength shifts when it binds to proteins with
a  -sheet structure. Biochemical
assay methods used in previous studies do not have structural specificityムas in
the case of using ThT. Therefore, these methods also detect aggregation of proteins
that do not have a -sheet structure. Biochemical
assay methods used in previous studies do not have structural specificityムas in
the case of using ThT. Therefore, these methods also detect aggregation of proteins
that do not have a  -sheet structure.
We considered that zinc suppresses -sheet structure.
We considered that zinc suppresses  -aggregation
of Ab, that is, the formation of fibrils, by accelerating the aggregation of proteins
that do not have a b-sheet structure. This was indicated when finding that A -aggregation
of Ab, that is, the formation of fibrils, by accelerating the aggregation of proteins
that do not have a b-sheet structure. This was indicated when finding that A aggregates formed in the presence of zinc were non-fibrous, different from those
formed in the absence of zinc (Fig. 1). We found that zinc suppresses A
aggregates formed in the presence of zinc were non-fibrous, different from those
formed in the absence of zinc (Fig. 1). We found that zinc suppresses A fibril formation by inducing formation of non-fibrous A
fibril formation by inducing formation of non-fibrous A aggregates.
aggregates.
While A fibrils are cytotoxic,
we investigated the cytotoxity of nonfibrous A fibrils are cytotoxic,
we investigated the cytotoxity of nonfibrous A aggregates formed in the presence of zinc. As a result, the nonfibrous A
aggregates formed in the presence of zinc. As a result, the nonfibrous A aggregates formed in the presence of zinc are less cytotoxic than fibrous A
aggregates formed in the presence of zinc are less cytotoxic than fibrous A aggregates formed in the absence of zinc (Fig. 2).
aggregates formed in the absence of zinc (Fig. 2).
Based on these results, the contradiction between epidemiological and biochemical
findings was resolved and we were able to clarify the suppressive effects of zinc
on both  -aggregation of A -aggregation of A and senile plaque formation. However, in order to determine the degree of effectiveness
of zinc in the prevention and treatment of AD, further verification is required.
Moreover, we have to investigate the role of zinc at the onset of AD. Further
examinations of the effect of zinc, by conducting pathological or behavioral experiments
using a mouse model exhibiting senile-plaque-like accumulation of A
and senile plaque formation. However, in order to determine the degree of effectiveness
of zinc in the prevention and treatment of AD, further verification is required.
Moreover, we have to investigate the role of zinc at the onset of AD. Further
examinations of the effect of zinc, by conducting pathological or behavioral experiments
using a mouse model exhibiting senile-plaque-like accumulation of A ,
are planned for future study. ,
are planned for future study. |
J. Biol. Chem., Vol. 276, 34, pp.32293-32299 (2001)
| |
 |
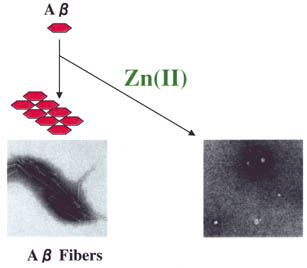 |
| Fig.
1 |
A aggregates in the presence
or absence of zinc. aggregates in the presence
or absence of zinc. |
| |
 |
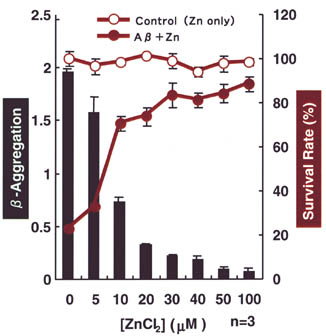 |
| Fig.
2 |
Zinc dose-dependent suppression of  -aggregation
and cytotoxicity of A -aggregation
and cytotoxicity of A . .
As the concentration of Zinc increases,b-aggregation is suppressed (bar graphs)
and cell-viability is preserved at high level (line graphs). |
|
 |
 |
|
|