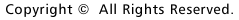|
magnified
scene by clicking image
| Figure
1. Schematic diagram of migration of a newly born neuron in the cerebral cortex, according to the former hypothesis. |
magnified
scene by clicking image
| Figure
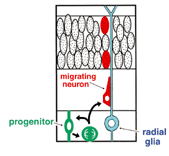
2. Division of RGC and birth of neuron. Time lapse record of fluorescence images taken from one DiI-tagged RGC taken from the meningeal surface side. The RGC performed cell division in the VZ in 1.7 to 6.1 hours, and gave birth to two daughter cells. One daughter cell inherited the parent's radial glial process (red arrow), and then migrated to the pia side following the retraction of the apical process (green arrow) to differentiate into the neuron. |
magnified
scene by clicking image
| Figure
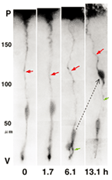
3. Birth of the neuron and progenitor from the RGC. Two daughter cells were born from the RGC in 6 to 9.2 hours. One inherited the parent's radial process (black arrow), and then retracted the apical process (green arrow head), migrated to the pia side and differentiated into the neuron. The other daughter cell (blue arrow) extended a new basal process (red arrow head) to the pia side, and after its tip end reached the border surface, underwent cell division again to give birth to two daughter cells. |
magnified
scene by clicking image
| Figure
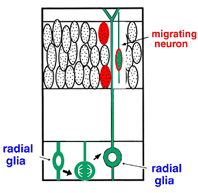
4. New schematic diagram of birth and migration of neuron |
magnified
scene by clicking image
| Figure
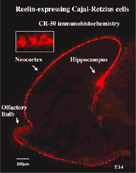
5. Cajal-Retzius cell. The Cajal-Retzius cell is the neuron appearing at the pallium first, and is deposited in the marginal zone. The layer alignment of the cortex neurons is controlled by the Reelin molecule, which is exclusively produced and secreted by these cells. |
|
|
Foreword
Cerebral cortex consists of six layers arranged parallel to the meningeal surface, each layer containing a different type of neuron, both in terms of form and function. From observations of various mouse mutants and human patients with congenital cranial, it is clear that this orderly layout of the neurons is the basis for physiological cerebral function. Understanding the principles behind the formation of cerebral structure is important for understanding the pathology of human diseases, and for progress towards regenerative medical care. Using cell and tissue culture, our team studies the birth and migration of cortical neurons in cortex, and the mechanisms by which the six-layered structure is formed.
Birth and migration of corticalneurons
How is a neuron born in cerebral cortex, and how does it migrate and differentiate? Many neurobiologists subscribe to the radial glial guidance theory, put forth by Rakic in the 1970s, which explains how migration occurs at the same time as the birth of a neuron. The basic gist of this theory is that the neurons are generated as free cells in the ventricular zone (VZ), then contact adjacent radial glial cells (RGCs). They then migrate to the cerebral meninges along the radial glial processes of the RGCs (Fig.1). It was believed that RGCs have their cell bodies in the VZ, traversing the cerebral wall with long radial glial processes (rgp's) that extend to the meningel surface from the cell body, and short apical processes that reach the lumen surface. It was also believed that they are static cells, their proliferation ending as cortical neurons are generated in the VZ. We tagged RGCs from the meningeal surface of embryonic mouse brain (E13 to 14) with a very small DiI crystal. We then observed and recorded the cell behavior of the live RGCs under a fluorescence microscope in real time or time lapse in our custom-developed three-dimensional slice culture. As a result, we showed that the RGCs were not static cells, but underwent cell division, generating neurons and progenitors, P. Specifically, we found that RGCs belonged to the class of cells including neuroepithelial cells. Upon cell division, the rgp of the parent did not extinguish, but was inherited to one of the child cells (Fig.2). We showed that the generated neuron which inherited the parent's rgp first had nuclear translocation to the cerebral meninges in the rgp, then migrated to the cerebral meninges following weaning of the apical process from the cerebral ventricles and reduction of the rgp. Meanwhile, the P cell generated by division of the RGC extended its basal process immediately. After reaching the meningeal surface, it underwent cell division again to generate granddaughter cells (Fig.3). These observations suggest that the newly generated neuron and the RGC, which had been considered to be from different cell lineages, are actually from the same cell lineage, and that when a neuron is born, it retains the specific cell form of the RGC and follows its peculiar autonomous gene information. These new concepts concerning the birth and migration of cortical neurons are compiled in recent special issues of "Cerebral Cortex" and" Glia". If you are interested, please refer to them. Development of the three-dimensional culture of cerebral wall tissue like ours made it possible for the first time to observe the real drama of the birth of cortical neurons and to watch the birth of new concepts in neuronal birth.
Formation mechanism of the six-layer structure of cerebral cortex
Cerebral neocortex constitutes of the six layers, with a different type of neuron, both in terms of form and function, in each one. Neurons within the same layer are born in the VZ at the same period, those born early are located on the inside of the layer, while those born later are located towards the outside of the layer (inside-out). Through joint research with the Mikoshiba Laboratory, we have clearly shown that the spatial alignment of cortical neurons is controlled by Reelin peculiarly secreted from the Cajal-Retzius cells, which appear first in the cortex, and are located in the marginal zone (Fig.5). In the mutant mouse reeler, the six-layer structure becomes abnormal (outside-in) due to deletion of this molecule. It has been clearly shown that the migrating cortex neuron receives signals through Reelin receptors such as apoER2, VLDLR, and CNR, and temporarily promotes tyrosine phosphorylation of Dabl of the intracellular adaptor molecule. In the Reelin-deficient mouse (reeler), the double deficient mouse of apoER2 and VLDLR, the Dabl-deficient mouse (yotari), and the mouse with the tyrosine residue of Dabl replaced with phenylalanine, similar abnormalities of the layer structure occur. However, it is not clear how this Reelin signal transmission is linked to motor molecules in the cell that actually related to the movement of the neuron. Incidentally, the fact that cortical neurons are deposited in an inside-out fashion means that neurons born later move to the outer layer. However, no one has yet succeeded in observing and recording later-born neurons born outrunning neurons born earlier during migration. We tagged migrating neurons born at different times with different fluorescence proteins and tried to record the dynamic migration of neurons in an improved three-dimensional culture.
Incidentally, in the human autosomal recessive inheritary Reelin disease, as reported recently, it was shown that the cerebrum develops lissencephaly in addition to aplasia of the cerebella. Rodent cerebra are smooth-surfaced to begin with, so it was not expected that lissencephaly would develop in the reeler mouse. However, the folia are not formed correctly in the cerebella of reeler mice. In the laboratory, we are currently investigating the mechanism by which the wrinkled structure of the cerebrum is formed, using the role of the Reelin in folium formation as a clue.
We have developed cell/tissue cultivation experiments systems effective in clarifying the principle of the tissue organization of the brain so far. In this manuscript, we introduced the birth and migration of neurons in cerebral cortex, and the mechanisms of layer formation, which examined using cultivated brain slices. Besides this, we have developed low cell density cultures, which are useful for analyzing the cell-lineages of neural and glial cells. We have also developed reaggregation cultures which are useful for examining cell adhesion between the cortex neurons, and we have also developed embryonic cerebellar organ cultures which are useful for studying cerebellar cortex formation. These experimental techniques were tested at various institutions here and abroad, yielding many results. As described before, we think that an important mission of our laboratory is to develop experimental techniques that support the research of other laboratories.
|
 |