The invisible touch
How do you jump-start the search for consciousness in the brain? A loan, some optical fibers and a cheeky threat of scientific one-upmanship
February 10, 2017
Amanda Alvarez
Cross-posted from Neurographic

Image: RIKEN Brain Science Institute
If you ask Masanori Murayama, the path to unlocking consciousness starts in the skin. The blanket of cells that covers the body is the most direct, keen connection we have to the world. “Shaking hands changes the surface tension of the skin,” observes Murayama, “while looking at someone’s face, for example, doesn’t change the face itself.” It is the act of touching and perceiving textures, or somatosensation, and the intimate link with the brain from the skin and the underlying muscle that is the object of Murayama’s ascending research career. He thinks if we can just describe how the brain perceives the world with sufficient accuracy — the underlying cellular activity and connections — we’ll have largely also found those elusive ‘neural correlates of consciousness’ (NCC), the brain signatures that define the awake, sentient state thought to be unique to higher mammals.
Murayama is nothing if not enterprising in this quest. He has built his own experimental equipment, relocated around the world for science, and even delivered newspapers at night to fund his studies. A tinkerer by nature, scientific questions for Murayama are not just about biology, but about how new technology can extend our senses to probe hidden realms. “We can’t study consciousness itself, but we can study the individual brain functions, like perception or decision-making or emotion, that make up consciousness,” says Murayama. “For me, perception seemed the easiest and most accessible.” The search for NCC is like reverse-engineering a Jenga tower. Scientists are trying to find the minimum number and configuration of supporting blocks that distinguishes the upright tower of consciousness from the toppled pile of blocks in unconscious states like sleep or anaesthesia. And when you’re looking for the basic building blocks of any scientific phenomenon, you have to start small and simple.
 |
The mouse brain is roughly the size of a pinky fingertip and has about 70 million neurons. Research on somatosensation in mice has traditionally focused on whiskers: stimulate a whisker, record the activity from the corresponding part of the brain called the barrel cortex which, like many perceptual areas of the brain, is structured like a map, neighboring whiskers represented by neighboring neurons. Murayama took a different tack, eschewing whiskers in favor of the mouse hindpaw. The stimulation of whiskers, says Murayama, may induce more than just sensory phenomena, since stimulated whiskers will subsequently move on their own, and active whisking will include motor signals. Paws and their representation in the brain are also more comparable to the whiskerless human experience. |
Image: Aleksey Pogrebnoj-Alexandroff
via Wikimedia Commons, CC BY
A puff of air to the mouse hindpaw launched Murayama’s quest to figure out the neural activity underlying consciousness, and in the past decade it has led him to the dendritic spike, an atypical electrical discharge generated within the cellular arbors at the receiving end of many neurons in mouse cortex. “The dendritic spike is a powerful computational element in the brain that occurs frequently in the awake, conscious state,” Murayama explains. “We think it has a crucial role in consciousness.”

Murayama in his lab in Japan circa 2011. Image: RIKEN Brain Science Institute
Now the laser-focused scientist, Murayama knows a thing or two about maintaining an awake state. When his teenage ambition of becoming a professional footballer fell apart in the wake of the Japanese economic bubble, he had some catching up to do, and in true Japanese fashion he headed to the cram school. To pay for all the intense after-hours studying, he delivered newspapers both day and night. During the day he staved off drowsiness by jabbing himself with a pencil, then returned to the newspaper offices to stuff ads in the papers.
It doesn’t seem accidental that Murayama’s research program now revisits some of these formative, intensely tactile themes. The air puff is just one in a suite of experimental sensory tests used in his lab to evoke responses in the mouse somatosensory cortex. In another touchy-feely task, mice explore different floor textures; making use of their natural attraction to novelty, researchers can test whether mice remember experiencing certain textures on previous days. The thinking is that accurate memory requires accurate conscious perception, which in turn relies on the right kind of brain cell activity. A string of studies published by Murayama and colleagues has zoomed in on the dendritic spike as the necessary signal for accurate perception. But finding the spike first involved an Alpine detour.
While Murayama was doing his PhD research in the early 2000s, researchers like Jackie Schiller started to report the discovery of a new kind of firing in brain cells. In addition to the conventional action potentials that originate in the central cell bodies of neurons, all-or-nothing spiking activity had been found also in the distant parts of neurons, the dendrites. At the time, the only research group that was successfully recording the electrical activity from multiple locations in a dendrite simultaneously was that of Matthew Larkum. Murayama was captivated: “I knew that was the place I had to go” to further his understanding of how these novel spikes influence the neural intricacies of perception.
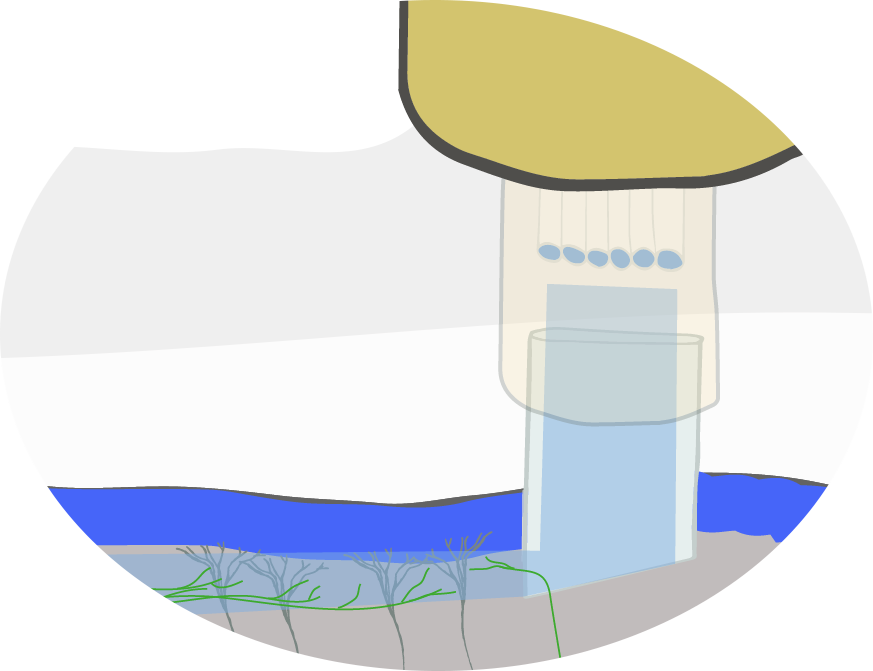
The ‘periscope’ used in Murayama’s research consists of custom microscope attached to a bundle of optical fibers and a right-angled prism that can direct light parallel to the surface of the brain, to both image from and stimulate the dendrites of pyramidal neurons.
Image: Adam Phillips/RIKEN
Larkum, now a professor at Humboldt University in Berlin, was based in Bern at the time. Murayama was in Tokyo. The money to make the move was nowhere to be found: Larkum couldn’t guarantee him a salary and Murayama’s grant applications were a bust. So he took a gamble. While finishing his PhD thesis, Murayama borrowed some money, splurged on optical fibers and lenses, and engineered a system for recording fluorescence signals in brain slices. Murayama called Larkum and told him about the equipment, his successful imaging of calcium ion activity, and reiterated his desire for a job in the lab. Then came the kicker: “I told him, you don’t have funding but I have a family, and if you can’t hire me, I will take my method to another lab.” Larkum got the head of the department to provide funding for Murayama, and in 2006 the family settled in Switzerland. “Matthew said passion is quite important,” chuckles Murayama, “and I demonstrated this passion.”
In Bern, Murayama was tasked with making the dendritic recordings possible in freely moving animals, rather than in brain tissue slices. He adapted his ‘periscope’ setup (see image above) so that the optical fibers could be used to both record from and deliver light to neurons while animals were sensing and behaving. Studying the sensory paw and motor brain signals of rats over the next few years, two things became apparent: brain activity in anesthetized and awake animals was radically different, with the latter containing a characteristic ‘late’ blip. Most of the activity reflected perceptual signals going ‘bottom-up’ from paw to brain in response to an air puff, but the blip, coming some tens of milliseconds after the main pulse of activity, hinted at delayed feedback from other brain areas. This has since come to be known as a ‘top-down’ circuit because areas further ‘up’ the anatomical hierarchy are sending output back down the line (see image below).
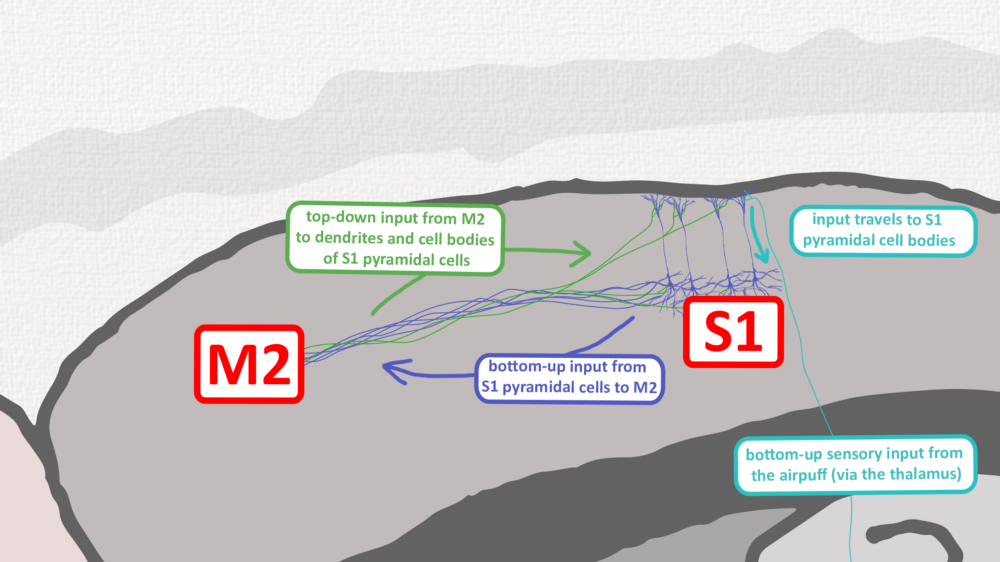
Touch signals from the skin travel up to the sensory cortex, and actions are controlled by the motor cortex, but these two brain areas are also reciprocally connected. This means that M2, the secondary motor cortex in mice, can send messages ‘top-down’ to its neighbor S1, the primary sensory cortex, a neural discussion that can lead to signals arriving simultaneously in the same neuron from two directions — an event that can lead to dendritic spiking. The feedback circuit between M2 and S1 not only modulates the incoming sensory inputs but is vital for mice forming accurate sensory perceptions and memories.
Image: Adam Phillips/RIKEN
There is still debate on what constitutes ‘higher’ and ‘lower’ areas, Murayama concedes, but the basic idea stems from how fast signals from the periphery of the body reach different brain areas. The thalamus, a central brain area that relays touch, vision and other sensory signals, gets hit first, then, with some delay, ‘higher’ areas receive the signals in turn. Conceptually, we also think of actions as coming after or as a result of having touched or seen something, and the anatomy and electrophysiology back this up: motor areas are the ‘top’ to the sensory cortex’s ‘down’.
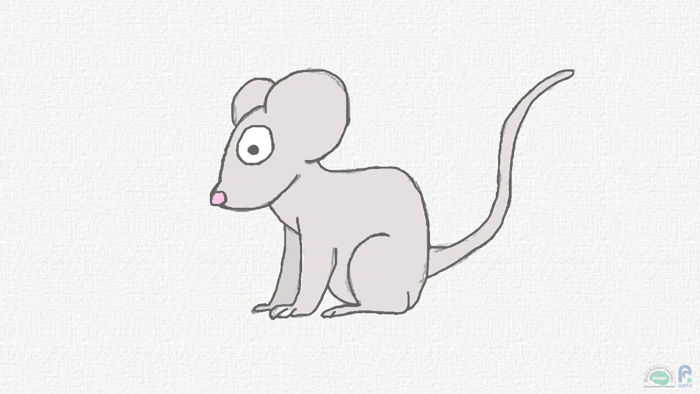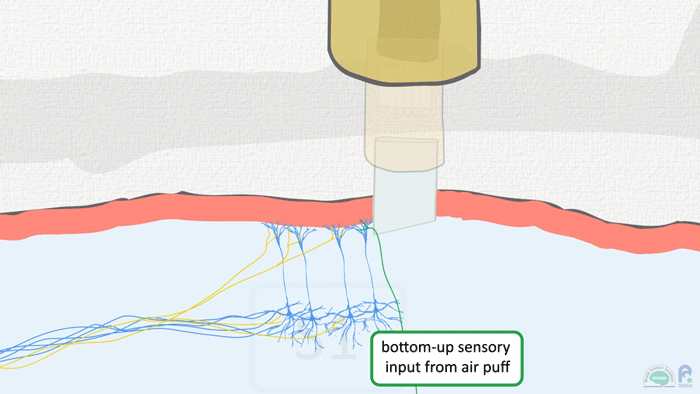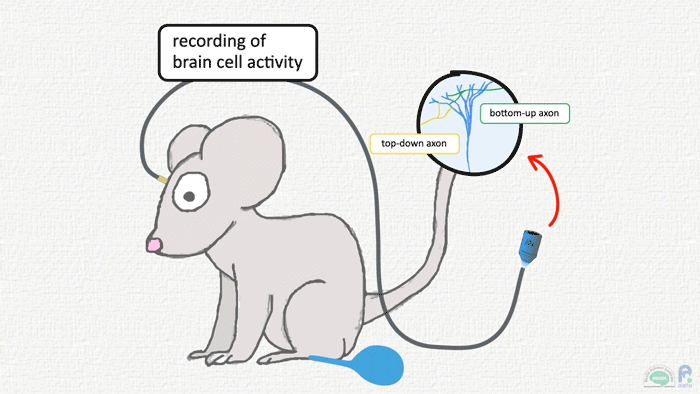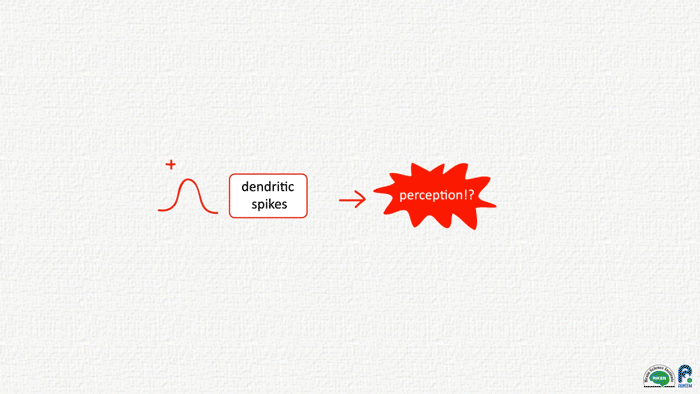
Image: Adam Phillips/RIKEN
Which brings us back to the dendritic spike. Murayama thinks he knows the significance of the top-down feedback between the adjacent brain areas: this ‘top-down coincident input’, as it’s been dubbed, is for generating a dendritic spike. These discharges on the nerve cell fringes cause the entire cell itself to start firing off repetitive action potentials. The fifth layer, the main output of sensory cortex, goes off like fireworks as a result.
These fireworks are essential for mice perceiving their environments accurately. Without the top-down input that kicks off the neural cascade, mice falter at sensory tasks, appearing to misperceive one texture for another. Likewise, activating or shutting down the top-down circuit during sleep, Murayama and colleagues discovered, could also enhance or undermine the persistence of perceptual memories in mice. Memories of learned environments, for example, were impaired in the absence of top-down signaling.
Accurate sensory perception in mice depends on a top-down cortical circuit; read the scientific paper here
Equivalent top-down control circuits in people are also not unheard of. The supplementary motor area in humans is thought to be analogous to the secondary motor cortex, or M2, of mice. Damage there can result in somatic neglect, leading to people becoming unaware of the corresponding part of the body. Shaving only half of your face may not be quite the same as being unconscious, but Murayama feels that, like the memory loss in his lab mice, local perceptual neglect is definitely indicative of how consciousness more broadly is formed and maintained.
The notion of top-down control may evoke visions of an inner puppeteer pulling the strings, a homunculus in the Cartesian theater of the mind. There is no ‘little man’ sitting inside watching the show, says Murayama, but there is a process assigning attention, value and memories to incoming sensory information — the top-down signal, for which dendritic spikes in pyramidal neurons appear to be essential.
To really clinch the story of conscious perception Murayama continues to probe the sensory cortex with self-engineered tools. His current undertaking is a powerful new ‘megascope’, a microscope with greatly enhanced field of view and resolution. Other than observing cells, manipulating dendritic spikes themselves would be the real kicker, but this requires some genetic engineering; you can’t turn the spikes on and off at will with light because as of yet no corresponding gene that is localized only in the dendrites that could be made light-sensitive has been identified. It’s clear that the cellular story of consciousness is far from finished and that Murayama will have plenty of research to occupy the coming years — the scientific explanation for consciousness may be within grasp.
Related Post
![]() RIKEN Press Release: Switching off brain circuit renders mice “out of touch” with environment
RIKEN Press Release: Switching off brain circuit renders mice “out of touch” with environment