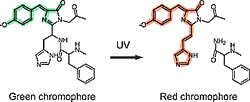
Isolated from a stony coral (Trachyphyllia geoffroyi), the cloned fluorescent protein Kaede (named for the Japanese maple tree) changes from green to red when exposed to ultraviolet light. Technology for marking cells that exploits this characteristic has been developed by researchers in the Laboratory for Cell Function Dynamics. They used this technology to observe and identify individual neurons within a neural circuit and to track the development of cells over time.
To understand how Kaede's conversion occurs, the laboratory conducted structural analyses of the protein in its green and red states. They discovered that the color conversion of Kaede's chromophore*1 occurs when nearby peptide chains break down and induce structural changes. This break down occurs when a catalytic reaction is triggered in the protein following exposure to ultraviolet light. This discovery demonstrates a novel photochemical property of proteins. Combined with laser technology, these results should lead to the development of new technology for manipulating the behavior of protein molecules using light.
Background
This fluorescent protein was cloned from a stony coral purchased in downtown Tokyo. It originally emitted a bright green fluorescence (maximum excitation 508 nm, maximal fluorescence 518 nm). However, when a sample was accidentally left overnight on a laboratory bench near a window, its color changed to a deep red (maximum excitation 572 nm, maximal fluorescence 582 nm). Analysis of the sample showed that the protein exhibited a wavelength conversion that responded to UV rays (photoconversion*2). We named this protein Kaede because it changes color from green to red.
The primary structure of Kaede has a certain degree of homology with already-known fluorescent proteins. However, Kaede has three amino acid arrays beginning with a histidine* 3 residue that serve as the foundation of its chromophore. The laboratory has subsequently developed technology for marking cells, intracellular organelles and molecules that take advantage of Kaede's photoconversion properties.
Results obtained
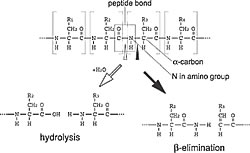
What mechanism enables Kaede's conversion from green to red when exposed to UV light? By comparing the green and red states of Kaede and analyzing the protein structure of each, we found that the Kaede-red peptide chain is cleaved near the amino acid extremity of the chromophore. This is the first report to cite that a specific wavelength of light induces peptide cleavage at a specific position. A b-elimination reaction cleaves the bond between the alpha carbon and the amide nitrogen in the histidine residue (Fig. 1).
This was astonishing because a large amount energy is required to break this particular bond. As a hydrolysis reaction in the peptide bond usually breaks a peptide chain, this break in Kaede may seem incredible (Fig. 2). However, the reaction formula does indicate that the phenomenon occurs in the protein. We concluded that the absorption of UV light acts as the catalyst for the peptide break in Kaede. We hypothesize that the catalytic action is enabled by a mechanism in the histidine residue in which the imidazole ring*4 supplies protons at the breaking position. Confirming this hypothesis should enhance our knowledge of the histidine residue's role in photochemical reactions.
This peptide chain break is a new phenomenon in two ways. First it is dependent on the absorption of UV light and second the reaction is not located at the peptide bond. Kaede's photoconversion from green to red indicates the completion of the reactions because the red fluorescence occurs only after the imidazole ring participates in the π-conjugation structure of the chromophore.
Future expectations
Kaede's conversion is an interesting phenomena, grasping the mechanisms behind this transformation will further our understanding of the interaction between light and proteins. Such investigations will also contribute to our knowledge on photochemical reactions within proteins. To this end, we are currently addressing two issues related to Kaede:
1. Is it possible to develop new nanotechnology that makes use of the breaking of the peptide chain by UV light?
2.Why does T. geoffroy's color change when exposed to solar light?