|
 |
 |
 |
Roles of Hippocampal NMDA Receptors
in Learning and Memory
Group
Director,
RIKEN-MIT Neuroscience
Research Center |
| Dr.
Susumu Tonegawa |
|
 |
 magnified scene by clicking image
magnified scene by clicking image
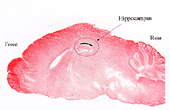
Fig. 1
WGA
transgene method for selective labeling of neural pathways of interest
By using WGA cDNA as a transgene in combination with a neuron type-specific promotor
element, anatomically-connected and functionally-related neural pathways can be
clearly visualized with great accuracy and high reproducibility. |
I have always been fascinated
by the so-called mind-brain problem. To what extent can we understand the mind
by studying the brain? For much of my research career, my expertise was in molecular
biology and immunology, and I knew almost nothing about neuroscience. My virtual
interest in neuroscience began to be transformed to a real interest when Alcino
Silva joined my lab as a postdoctoral fellow in the late 1980s. At that time our
lab was working on problems in immunology, and we were making genetically altered
mice. While discussing possible research projects with Alcino, I realized that
he too had a hidden interest in neuroscience. As far as we knew, no one had attempted
to apply powerful genetic engineering technology to neuroscience.
magnified scene by clicking image
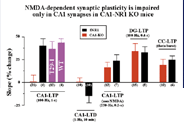
Fig.
2 The picture shows that in the CA1-specific NR1 knockout mice NMDA receptor-dependent
synaptic plasticity (LTP and LTD) is impaired in CA1 synapses but not in dentate
gyrus (DG) or somatosensory cortex (CC) synapses. T29-1 and WT are the Cre transgenic
and wild-type mice, respectively, while fNR1 is a mouse into whose genome a pair
of LoxP sites are inserted upstream and downstream of the NR1 gene. |
To decipher events occuring
in the brain that underlie a cognitive phenomenon, one must select an experimental
method that uses a whole, live animal. It seemed clear that knockout mice had
the potential to be a very powerful tool. In knockout mice, a specific gene is
deleted, so any cognitive or behavioral deficits observed in these mice relative
to normal mice can directly or indirectly be attributed to the lack of a single
gene product. A similar approach had been used by others to study the molecular
basis of invertebrate behaviors, but work along these lines had never been applied
to vertebrates, certainly not to mammals.
We decided to study learning and memory as the cognitive function and chose the
a form of CaMKII as the target for knockout. In 1992, this work led to the report
of the first knockout mice in neuroscience. It was shown that deletion of the
a-CaMKII gene causes a deficit in LTP at Schaffer collateral-CA1 synapses and
an impairment of spatial learning. Even before publication, however, we were aware
of the limitations of this approach in conventional knockout mice. These limitations
were primarily because the gene of interest in deleted in the entire animal throughout
the animal's life. Although not obvious developmental defects were observed in
the a-CaMKII knockout mice, more subtle defects could not be excluded. Furthermore,
the universal absence of the protein in question (in this case, a-CaMKII) certainly
did not permit the establishment of a causal relationship between CA1 LTP and
spatial learning.
 magnified scene by clicking
image
magnified scene by clicking
image
Fig. 3
Three dimensional graphs representing occupancy of normal and (CA1-specific NR1)
knockout mice during the probe trial session of the hidden platform version of
Morris Water Maze task. The control mice focused their search in the trained location
(where the platform used to be during training) whereas the mutant mice visited
the whole maze area equivalently. |
When Joe Tsien, another
postdoctoral fellow, arrived in my lab in 1993, we started to develop a second-generation
gene knockout technology that would restrict the deletion of specific gene to
a limited region of the brain. After 2 years of hard work, Joe generated several
lines of mice whose genetic changes were restricted to specific neurons in the
forebrain. We were thrilled to find that in one of these lines of mice, changes
were restricted to the CA1 region of hippocampus. Joe went on to use this line
to produce a new mouse strain in which deletion of the NMDA receptor gene, NR1,
was restricted to the CA1 pyramidal cells. (Fig. 1) Joe and Pato Huerta, another
postdoctoral fellow, demonstrated that in these mice, LTP and LTD are deficient
at Schaffer collateral-CA1 synapses but are normal elsewhere, such as perforant
path dentate granular cell synapses (Fig. 2). They also showed that the mutant
mice are deficient in hippocampus-dependent learning (Fig. 3). Furthermore, a
collaboration with Matthew Wilson's laboratory across the hall demonstrated that
these mutant animals could not form normal place fields in CA1 (Fig. 4). All of
these data provided very strong evidence for Hebb's hypothesis on the synaptic
bases of memory formation.
I informed Alcino that we seemed to have developed a technology to restrict NR1
gene knockout to the CA1 area, and the ever cheerful and enthusiastic Alcino exclaimed
"Wow! God Given! Congratulations!" CA1 is a major anatomical region in the hippocampus
that had been implicated in memory formation, and the Schaffer collateral-CA1
synapse may indeed be regarded as a God-given gift.

magnified scene by clicking image
Fig.
4 Place fields of NMDAR1 CA1-KO mice are significantly larger in all behavioral
environments. Rate maps of place-specific activity of two pyramidal cells from
control animals and two pyramidal cells from knockout animals in each behavioral
environment. The peak rate of each panel has been adjusted to reveal areas of
highest activity. The field sizes of the pyramidal cells of the NMDAR1 CA1-KO
animals were significantly larger in both the linear track (one-dimensional) environments
and the open field (two-dimensional) environment. |
Can we develop technology
that would target gene manipulation to other specific brain areas or cell types?
Recent studies in our laboratory indicate that this is indeed possible. For instance
Kazu Nakazawa, another postdoctoral fellow in my laboratory, has recently succeeded
in developing a method to target gene knockout to the CA3 pyramidal cells of the
post-developmental hippocampus. With this method Kazu created CA3-specific NR1
knockout mice. In contrast to the CA1-specific NR1 knockout mice these new mutant
mice are normal in hippocampus-dependent learning. However, they exhibit a severe
impairment during the process of memory retrieval. The properties of place cells
formed by the CA3-specific NR1 knockout mice fit nicely to the findings observed
at the behavioral level. We can conclude that the same glutamate receptor (i.e.
NMDA receptor) plays very different roles in the mnemonic process depending on
in which type of hippocampal cells it is expressed. Nothing illustrates more dramatically
than these studies the importance of the specifically restricted knockout mice
for the study of learning and memory and, by extrapolation, other cognitive functions.
With the addition of the emerging techniques that would permit reversible temporal
control of gene manipulation, rodent behavioral genetics is poised to become a
powerful approach for the dissection of mechanisms underlying cognition and behavior.
|
 |
 |
|
|