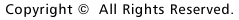|
 |
 |
Hypophagic
and Lean Gene Information which Controls Appetite
Laboratory for Cell
Culture Development |
 |
 |
 |
| Fig.
1 |
We showed that appetite,
the most important drive in living organisms, is genetically controlled and is
not associated with one's consciousness. The hypothalamus plays important roles
in life maintenance such as regulating the body temperature and food intake. It
has been recognized that "appetite information" based on the levels of sugar,
insulin and leptin, a hormone secreted by adipocytes, in the blood is transmitted
from the peripheral tissues to the hypothalamus which in turn transmits "appetite
information" through neurotransmitter substances in the brain. We have clarified
for the first time that the acetylcholine neurotransmitter acts on this transmission
mechanism, using muscarinic receptor. The muscarinic receptor, one of the receptors
which transmit the information of this neurotransmitter substance acetylcholine,
has five different subtypes (M1-M5), and it is known that each subtype is expressed
in the brain. It seemed that the physiological functions of these subtypes had
been elucidated completely by extensive pharmacological analysis. However, the
specificity of an antagonist in selectively matching to a particular subtype is
not absolute. Furthermore, it is disadvantageous that the specificity of an antagonist
is lost when present at high concentrations. Therefore, the specific physiological
functions of the subtypes remained unclarified.
To address this issue, Cell Culture Development team (Masaharu Ogawa team leader)
and National Institutes of Health research group (US) collaborated to generate
mice that lack the gene for each muscarinic receptor subtype and analyzed the
specific physiological functions of these subtypes.
In the mice that lack the gene for M3 muscarinic receptor, reduction in body weight
(about 23% reduction in the body weight) which was caused by the reduction in
food consumption (about 25% reduction) was observed; this characteristic was not
observed in other mice lacking other genes for other subtypes of the muscarinic
receptor. Moreover, the amount of adipose tissue in the mice decreased markedly
compared with that in the other mature mice even though their body lengths were
the same (Fig. 1). Blood test results indicate that the amount of leptin in blood,
which originated from the adipose tissue, decreased markedly. Although the reduction
in the leptin level was information from the peripheral tissue indicating "hunger",
paradoxically the M3-lacking mouse exhibit hypophagic. This mouse did not exhibit
anything unusual in its physical and metabolic activities, or learning ability
which require high-stage functions of the brain. These results indicate that appetite
information was blocked in the central nervous system of the M3-lacking mouse.
In fact, the M3-lacking mouse did not respond to the administration of a neurotransmitter
substance, AGRP, which promotes appetite inside the brain. This suggests that
the information was blocked in melanin-concentrating hormone (MCH)-producing neurons
on which the AGRP receptor is expressed (Fig. 2).
To date, it is difficult to develop a functional inhibitor that acts on each muscarinic
subtype, because the receptor proteins of the subtypes of muscarinic receptors
are quite similar in structure. By analyzing the function of receptor subtypes
at the gene level, we clarified the mechanism through which muscarinic receptor
subtypes maintain certain life processes, which was a target of our study. Such
a genetic approach is considered to lead to efficient development of novel medicines
that are effective against the target receptor subtype, as well as an effective
method for exploring new physiological functions of the subtypes. We expect that
significance of the genetic approach will increase further in the future.
|
 |
 |
|
Fig.
2
The route of eating stimulus in the hypothalamus and eating behavior following
the intra-brain administration of neurotransmitter substances which stimulate
appetite. Leptin is secreted from adipocytes when satisfied with food, enters
the blood circulation and binds with the leptin receptor that exists in the arcuate
nucleus (ARC). This results in the synthesis and secretion of melanocortyn which
competes with (agouti-related peptide) AGRP, a neurotransmitter substance which
enhances the appetite. This in turn inhibits synthesis and secretion of MCH resulting
in the control of eating behavior. Our functional analysis reveals that the blockade
of the neural circuitry occurs in MCH-producing neurons of the M3-lacking mouse.
The M3-lacking mouse does not respond to AGRP, but does respond to orexin and
MCH, which are involved in downstream of the neural circuitry and took food, similar
to the control group. Results of this experiment indicate that the transmission
of information regarding "hunger" from the peripheral tissues is interrupted because
of the insufficiency in the function of MCH-producing neurons in the lateral hypothalamic
area (LHA).
magnified scene by clicking image
M.
Yamada, T. Miyakawa, A. Duttaroy, A. Yamanaka, T. Moriguchi, R. Makita, M. Ogawa,
C.J. Chou, B. Xia, J.N. Crawley, C.C. Felder, C.-X Deng., J. Wess Mice lacking
the M3 muscarinic acetylcholine receptor are hypophagic and lean. Nature, 410,
pp207-212 (2001)
|
|
 |
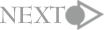 |
|
|