|
 |
 |
Untranslated
Region of Mu-Opioid-Receptor Gene Contributes to Morphine Sensitivity
Laboratory for Neurobiology
of Emotion
Laboratory for Cellular Information Processing |
 |
 |
Researchers
in the laboratories for Neurobiology of Emotion (Head, Dr. Hiroaki Niki) and Cellular
Information Processing (Head, Dr. Ryoji Yano,) in collaboration with the Brain
Research Institute of Niigata University, clarified that an untranslated region
in the mu-opioid-receptor gene affected the pain-killing efficacy of morphine.
This finding suggests the possibility that differences in the untranslated region
may be the genetic basis of individual differences in morphine sensitivity. We
expect that further studies on this possibility will lead to the creation of custom-made
medications in the future.
Morphine is the main component of opium which is extracted from the buds of the
poppy plant. Opium has been used for a long time by humans as a palliative drug
and narcotic. The opium poppy is even mentioned in Greek mythology. Morphine,
which is very effective in relieving pain and inducing pleasant feelings, is still
widely used today to reduce post-operative pain and as treatment for patients
with terminal cancer. Recently, the importance of controlling pain has become
seriously recognized in various clinical fields and the amount of morphine used
has increased annually. These trends indicate the significance of developing an
appropriate pain-relieving regimen for the use of morphine. In contrast to these
advantages, it is acknowledged that opium and morphine are narcotics which cause
physical and mental addiction. Therefore, morphine must be used under an appropriate
regimen with minimal side effects, but still a substantial pain-relieving efficacy.
However, morphine sensitivity greatly differs among individuals, and the bases
for such individual differences have not been clarified.
For this study, we utilized the widely held rationale that studying genetic mechanisms
of strain differences in mice is an effective way to investigate the genetic bases
of individual differences in humans. Therefore, we studied the genetic bases of
pain-relieving mechanisms in the CXBK mouse model, which is known for having a
low responce to the pain-relieving efficacy of morphine. First, we clarified that
in mu-opioid-receptor genes in the CXBK mouse abnormalities were observed not
in the region which determines the protein structure (coding region), but in the
untranslated region which remains largely unknown (Fig. 1). The mu-opioid-receptor
in the brain is the target of morphine. Next, we clarified that certain characteristics
of CXBK mice are caused by mu-opioid-receptor genes of CXBK mice (Fig. 2). As
shown in Fig. 2, mice (CX ) with
only mu-opioid-receptor genes derived from CXBK mice (CX), mice (B6 ) with
only mu-opioid-receptor genes derived from CXBK mice (CX), mice (B6 ) with only mu-opioid-receptor genes derived from normal mice (B6), and mice (He
) with only mu-opioid-receptor genes derived from normal mice (B6), and mice (He ) with one mu-opioid-receptor gene derived from CX and one from B6 were generated.
All of the mice were littermates. In CX
) with one mu-opioid-receptor gene derived from CX and one from B6 were generated.
All of the mice were littermates. In CX mice, the untranslated region of the mu-opioid-receptor mRNA was longer and expressed
at lower levels than that in its siblings. The efficacy of morphine was also lowest
in CX
mice, the untranslated region of the mu-opioid-receptor mRNA was longer and expressed
at lower levels than that in its siblings. The efficacy of morphine was also lowest
in CX mice, which was the same level
as observed in CXBK mice. mice, which was the same level
as observed in CXBK mice.
It is reported that differences in the untranslated region influence the stability
of mRNA, causing differences in the level of protein produced as a result of translation.
We therefore propose the following hypothesis: CXBK mice have a completely normal
mu-opioid-receptor, but since abnormalities are observed in the untranslated region,
the level of mu-opioid-receptor mRNA in the brain decreases, resulting in a reduction
in the level of the mu-opioid-receptor protein in the brain which in turn results
in the insufficient transmission of pain-relieving signals induced by morphine.
Consequently, the pain-relieving efficacy of morphine in CXBK mice is insufficient.
In addition, it was reported that unlike in the coding region, the DNA sequence
is not conserved in the untranslated region evolutionally and therefore, individual
differences are observed in the untranslated region. We consider the possibility
that differences in the untranslated region of the mu-opioid-receptor mRNA may
be one of the genetic bases of individual differences in morphine sensitivity.
Furthermore, we assume that not only in mu-opioid-receptor, but also in proteins
which govern various brain functions, individual differences in the amount of
protein expressed due to variations in the untranslated regions occur, resulting
in individual differences in brain functions. |
| |
 |
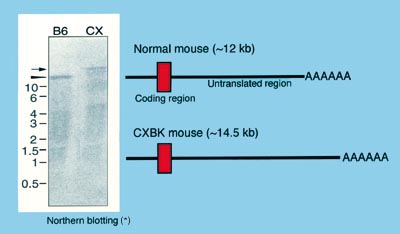
(*): Methods used to estimate length and level of mRNAs.
Fig. 1. In CXBK mice, the untranslated region of the mu-opioid-receptor mRNA is
long.
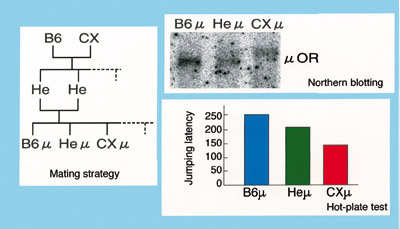
Fig. 2. Mu-opioid-receptor genes derived from the CXBK mouse contribute to a decrease
in the level of the mu-opioid-receptor proteins and morphine sensitivity. |
| Ikeda,
K., Kobayashi, T., Ichikawa, T., Kumanishi, T., Niki, H. and Yano, R., The untranslated
region of mu-opioid-receptor mRNA contributes to reduced opioid sensitivity in
CXBK mice, J. Neurosci., (2001)21: 1334-1339. |
|
 |
 |
|
|