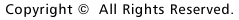|
magnified
scene by clicking image
| Fig.1 |
By
crossing a mouse that expresses recombinase in oligodendrocytes with a loxP-p35
mouse, DNA recombination specifically occurs in oligodendrocytes, resulting in
p35 expression. In the p35-expressing mouse, development of experimental autoimmune
encephalomyelitis (EAE) and inhibition of demyelination were observed. |
|
Introduction
Programmed cell death (often called "apoptosis"; however researchers in the fields
of genetics of developmental biology or invertebrates who study programmed cell
death prefer the term "programmed cell death") is a general mechanism of eliminating
unwanted cells generated by multicellular organisms. In terms of morphogenesis,
the role of programmed cell death is compared to the work for a sculptor to male
a form using chisels. It is noteworthy, however, that this phenomenon is particularly
frequently observed in tissues of the nervous and immune systems that are characterized
by the presence of various cell types that are not closely related to morphogenesis.
In the nervous system, programmed cell death which is directly related to morphogenesis
is observed during the initial development of the nervous system. However, after
this initial development, programmed cell death plays the function of cell selection
mechanisms with which cells to be eliminated are selected from a group of neurons
with various characteristics. Cell death during the development of the nervous
system proceeds as follows.
1. Cell death observed during the initial development of nervous system
Followed by neural induction, neural plate causes morphogenetic movements and
forms a neural tube by fusing the neuroepithelium. On the fused surface, cell
death is observed.
2. Cell death in ventricular zone of the cental nervous system
In the central nervous system (CNS), at the fetal stage, cells in the ventricular
zone actively undergo cell division, and produce all cells that will later form
the brain; mass cell death is observed in this cell group.
3. Cell death at the stage of synapse formation
At the stage of the neural network formation, neurons that can form synapses with
the appropriate targets survive, and those that cannot are eliminated by cell
death.
4. Cell death in the neurodegenerative disease
Mature neurons that contribute to the development of the neural network survive
throughout the lifetime of the organisms without undergoing cell division. Glia
cells, whose number is more than ten times of the neurons, support the longevity
of these mature neurons. However, a certain number of neurons die with aging,
which is believed to reach one hundred thousand per day in healthy humans. When
the individual suffers from a neurodegenerative disease such as Alzheimerユs disease
or Parkinsonユs disease, neuronal death in a specific area of the brain is accelerated,
resulting in the development of serious neurodegenerative symptoms. In our laboratory,
we conduct studies in order to explore the biological significance of neuronal
death and to develop methods of inhibiting neurodegeneration by clarifying the
basic mechanisms of neuronal death.
Beginning
of the genetics of cell death; impact of the genetic studies in nemadode C. elegans
The complete description
of the cell lineage of the nematode Caenorhabditis elegans (C. elegans) has brought
the great impact to the study of cell death. One thousand and ninety somatic cells
are produced during the development, among which 131 cells are lost due to cell
death at specific development stages (this cell death is particularly frequently
observed in the nervous system, and 105 of the 131 cell deaths occur in the nervous
system). Since a mutant gene (ced-3 mutant) which inhibits the death of all 131
cells has been identified, the presence of genes which regulate programmed cell
death is suggested. Programmed cell death itself is a general phenomenon observed
in various species, and therefore I considered that similar cell-death-executing
genes should exist in mammals, and decided to enter this field of study.
magnified
scene by clicking image
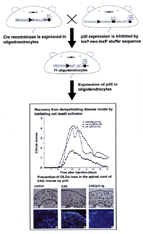
| Fig.2 |
In
larvae with the loss of function mutation of caspase activating factor Apaf-1,
hypertrophy of brain and inhibition of cell death were observed. On the other
hand, overexpression of the cell-death-promoting factor, the Bax-type Bcl-2 family,
Drob-1, in a compound eye causes neurodegeneration. The strategy of our study
is to comprehensively identify cell-death-promoting genes in Drosophila and apply
the knowledge obtained to the understanding of mammalian neuronal death. |
|
Cell-death-executing
genes of mammals
Subsequent to the identification
of ced-3, a cell-death-executing gene of the C. elegans, I encountered a highly
exciting study which identified the cell-death-executing gene (ICE) in mammals
for the first time. Subsequent studies clarified that cell death genes similar
to ICE encode members of the protease family called caspase. Since caspase is
considered a common mediator of cell death, we inferred that neurodegeneration
could be inhibited by regulating caspase activity. Fourteen members of caspase
have already been identified in mammals. Our strategy was to use a caspase inhibitor
gene from baculovirus p35 gene which inhibits almost all of caspases. We expected
to inhibit most of caspase activity by expressing p35 in the neural tissues. Multiple
sclerosis is a representative autoimmune disease of the CNS in which oligodendrocytes
specifically degenerate. No efficient strategies of treatment has been established
(a famous cellist, Jacqueline Du Pr・ died of this disease). We succeeded in inhibiting
the development and progression of multiple sclerosis by expressing the caspase
inhibitor gene p35 in oligodendrocytes in a mouse disease model (Fig. 1). A study
of the regulating mechanism of genes which execute programmed cell death was initiated
using the C. elegans, which would possibly enable the development of a method
of treating neurodegenerative diseases in mammals.
Nobel
genetic approaches to explore the neural cell death
Programmed cell death
and neurodegeneration occur in the brain, a most complex organ in human. Ideally,
cell-death-regulating factors present in the brain should be explored by a genetic
screening method because neurodegeneration must be regulated by various cell to
cell interactions in the brain. For this purpose, we use Drosophila as the model
organism. By taking advantages of Drosophila genome project, cell-death-regulating
factors in the mutant which were obtained by genetic screening could be quickly
identified. Furthermore, we developed a model similar to the neurodegenerative
diseases in humans, bringing about the possibility that genetic studies of neurodegenerative
disease, which are difficult to conduct in mammals because of the long period
until symptoms develop, may be carried out using Drosophila. Based on our previous
studies, it was clarified that a caspase-activated mechanism is conserved in humans
and Drosophila, and a caspase activating factor called Apaf-1 plays a significant
role in regulating the death of undifferentiated neuronal cells particularly during
the early developmental stages in Drosophila (Fig. 2). We also clarified that
a Drosophila homologue of the Bax type Bcl-2 family genes, named Drob-1, induces
neuronal death independent of caspase (Fig. 2). It is suggested that cell death
independent of caspase is related to mammalian neurodegeneration in various aspects.
However, its genetic regulation is completely unclarified, and we thus aim to
undertake this task. Completion of the Drosophila genome project enables us to
explore the cell death genes comprehensively in Drosophila. We are now standing
at the unique position to perform studies using mouse concurrently with studies
using Drosophila. By genetically exploring the entire picture of the yet-to-be-clarified
neuronal-death-executing mechanism in Drosophila and applying the obtained knowledge
to the understanding of human neuronal death, we hope to develop a new treatment
for neurodegenerative diseases (Fig. 2).
|
 |
 |
|
 |
|
|