|
Introduction
Among research
groups in the Brain Search Institute (BSI), the Advanced Technology Development
Center (ATDC) is unique. There are no technology-oriented research groups like
ATDC in the world. Since technological development is essential in achieving great
advances in the field of brain science, ATDC was established to promote the development
and application of research technology from the aspects of both hardware and software
technologies, including improvement of facilities. In addition, through joint
research collaborations with other organizations, ATDC aims to effectively transfer
technology both within BSI and to non-BSI researchers in order to support them
in their research efforts.
Our team for the development of nerve construction technology is involved in the
improvement and development of microscopic analysis technology, and actively participates
in joint research projects through its involvement in morphological analysis of
various research projects. The following are our priorities with respect to technological
development: 1) specimen preparation technology, which selectively visualizes
cell components comprising nerve tissues and neural circuits, 2) technology for
microscopic analysis of localization of intracellular functional/component molecules
and the morphology of these molecules, and 3) technology for highly accurate analysis
of the functional structure of cells comprising synapses. Just as the telescope
is essential for astronomical observation, so is the microscope a reliable tool
for observing the structure of the intrabrain, otherwise called the microcosm.
We analyze brain structures that regulate brain functions using the latest microscopic
observation techniques.
magnified
scene by clicking image
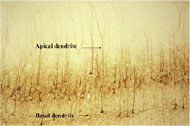
| Fig.A |
Immunostained
image of pyramidal cells of the cerebral cortex. From a soma, one apical dendrite
extends in the vertical direction, and many basal dendrites extend in the horizontal
direction. |
magnified
scene by clicking image
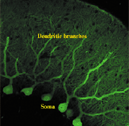
| Fig.B |
Purkinje
cells of the cerebellar cortex. Axons that extend in a fan-shaped manner from
soma ramify, similar to a dense forest. |
|
Components
of nerve tissuesneurons and gliacytes
For our first
research topic, "Selective Visualization of Components of Nerve Tissues," we used
both conventional optical microscopic observation technology and a laser scanning
microscope.
The brain consists of two types of cells, namely, neurons and glia. Neurons process
information transmitted through nerves by forming a complex circuit network, whereas
glia support the function of neurons. In the brain, these cells are arranged systematically.
Thus, the localization and arrangement of these cells and how they assemble to
comprise nerve circuits need to be studied in detail in order to obtain a complete
picture of the brain.
To observe the complex structure of the intrabrain using a microscope, appropriate
specimens, optimal for the above-mentioned purpose, must be prepared. In general,
thinly sliced specimens are developed for histostaining in order to study elements
comprising the tissue, which are then subsequently subjected to microscopic observation.
One of our frequently employed methods for preparing specimens is immunohistochemistry,
which enables visualization of protein molecules specific to cells using an antigen-antibody
reaction and detection of particular types of cells in the brain. Through a combination
of the intracellular staining technique and immunohistochemistry, we aim to clarify
the mechanisms underlying the formation of nerve circuits via different types
of cells, specifically, the basic mechanisms of cell arrangement to form nerve
circuits.
Neurons extend projections such as dendrites and axons originating from the soma,
which have morphological characteristics completely different than cells of other
organs in the body. The manner in which the projections are extended varies depending
on the type of neuron. In our study, the intrabrain structures such as the cerebral
cortex, the cerebellar cortex and the superior colliculus, which are formed in
layers, are targeted for microscopic morphological analysis. This is because we
are interested in the significance of the layered structure in localized nerve
circuits. Here, we describe results of the staining. For example, when pyramidal
cells are stained using the immunohistochemical method with the protein level
of neurotubules as an index, we observe that long apical dendrites are extended
toward the surface layer and basal dendrites are extended in all directions in
the area adjacent to the soma (Fig. A). When Purkinje cells in the cerebellar
cortex are stained using the immunohistochemical method with the calcium-binding
protein (calbindin) level as an index, unusual fan-shaped dendrites that extend
toward the surface layer appear (Fig. B). Since dendrites serve as an area in
which neurons transmit information, that is, information is received in synapses,
dendrites play a role as information antennae. Differences in the directions or
shapes of dendrites reflect differences in information accumulation and processing
functions of the cells. The specimens also contain axons that act as information
transmitters. However, it is difficult to clarify in which direction axons extend
for the following reasons: 1) they are extremely thin, 2) they can only be observed
under high magnification, and 3) they are highly entangled since they extend from
numerous cells; thus, a typical specimen is not optimal for clarifying axons.
The tract-tracing technique is used to stain axons that communicate with an information
receiver, and will be described elsewhere.
Here, we employ some types of glial cell as specimens for observation. With the
recent developments in molecular biological research, the role of glia in neural
tissue is being reexamined. We are currently working towards determining marker
molecules of glial cells, developing an antibody for these marker molecules and
morphologically detecting only glialccells. We are also developing a specific
antibody against the thymosin protein which is a good marker of microglia, and
will apply this specific antibody to brains in the developing stage or with human
HuntingtonÕs disease, and analyze the behavior of microglia. The cerebellar cortex
contains Bergmann glia with unusual shapes. We will also aim to clarify protein
molecules which are observed only in this type of cell, develop a monoclonal antibody
and perform immunohistochemical analysis of Bergmann glia.
magnified
scene by clicking image
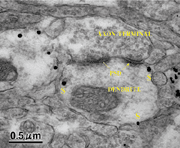
| Fig.C |
Immunoelectron
micrograph of a cell adhesion molecule, telencephalin. Black markers of this molecule (s) are observed in an area adjacent to the dendrite membrane, not in the postsynaptic density (PSD). |
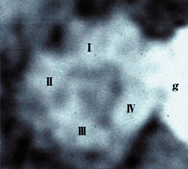
magnified
scene by clicking image
| Fig.D |
Electron
micrograph of one calcium channel molecule stained by colloidal gold (g). This molecule, with a diameter of approximately 10 nm, consists of four sections (I~IV).
A pore is formed at the center of four sections, through which calcium ions pass. |
|
Ultrastructural
analysis
The previously
mentioned second and third specimens require analysis by means of an electron
microscope. To analyze these specimens, we attempt to apply atomic force microscopy,
orginally developed for observation of engineering specimens, to the analysis
of neurons.
In order to observe the localization of intracellular molecules, immuno-electron
microscopy, in which samples are immunostained, is often employed. Cases detected
using this method are described below. One is our study on the localization of
telencephalin molecules which are specifically expressed in neurons in the forebrain
(Fig. C). Telencephalin is a cell adhesion molecule. However, it is an exceptional
adhesion molecule among known cells that are localized in dendrites but not in
axons. In Figure C, this molecule is shown immunostained with colloidal gold (diameter:
10 nm). Detailed observation reveals that a marker is observed not in the postsynaptic
density (PSD) but in its periphery. The function of this molecule remains unclarified.
However, results in Fig. C suggest that telencephalin may have a role different
from the direct role in synaptic-transmission. Another study employing immunoelectron
microscopy is the observation of calcium ion channel molecules in frozen fracture/replica
specimens (Fig. D). These molecules are P/Q-type channel molecules stained by
colloidal gold. Each of these molecules consists of four sections. A pore is formed
at the center of the four sections, through which calcium ions pass. These molecules
are a good example of morphological evidence of the structure of intracellular
molecules that were initially formed based on a molecular model.
To study the ultrafine structure of neurons using techniques other than the above-mentioned
immunoelectron microscopy, we used electron microscopic analytical technology,
including electron energy loss spectroscopy for localized analysis of various
elements inside tissues and scanning electron microscopy (SEM) for stereo structural
analysis.
Conclusion
To clarify mechanisms
of brain function, various research techniques, from the molecular level to even
a system or social science level (e.g. in vitro experiments, recording, behavior
analysis and construction of a theoretical model of nerve activities), are employed.
The functional structure theoretically determined by such an analysis will be
conclusive evidence of the functional structure if practically and morphologically
confirmed in the brain. We believe that clarification of the brain structures
which regulate its function and the development of morphological observation technologies
required for such clarification are significant in attaining our goal.
|
 |
|
|