|
 |
 |
Development
of a New Fluorescent Protein
that Enables Detailed Observation of Living Cells
Laboratory for Cell
Function Dynamics |
|
 |
1.
Background
Cellular phenomena such as differentiation, locomotion, and division manifest
along specific reaction paths inside of cells. A large number of the
functional molecules involved in transmitting signals inside of cells have been
biochemically or genetically identified. However, in order to gain a comprehensive
understanding of matters such as the signal transmission system, it is necessary
to observe phenomena inside of individual cells. To achieve this, fluorescent
labeling of targeted genes and other intracellular sites is carried out using
various substances, and it is necessary to design various techniques in order
to enable observations.
However, green fluorescent protein (GFP), which is one of the conventional fluorescent
labeling substances, is of low effectiveness in forming chromophores, and although
it is a protein, it is not capable of luminescence when immature. There is a pressing
need to observe subtle changes in fluorescence that occur locally in areas such
as intracellular organelles. Finding a GFP that effectively forms chromophores
is also required.
2. Regarding a new, modified GFP
We introduced random amino acid substitutions into modified GFP (approximately
240 amino acids) from Aequorea coerulescens. We paid particular attention to the
oxidation reaction, which is thought to be the most important of the reactions
associated with chromophore formation. At 37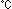 ,
the optimum culturing temperature for mammalian cells, we found that the chromophore
formation reaction hastened dramatically when we substituted leucine for the 46th
phenylalanine. We also identified amino acid substitutions that increase the effectiveness
of protein folding (Note 1), and ascertained that these accelerate the maturation
of GFP. ,
the optimum culturing temperature for mammalian cells, we found that the chromophore
formation reaction hastened dramatically when we substituted leucine for the 46th
phenylalanine. We also identified amino acid substitutions that increase the effectiveness
of protein folding (Note 1), and ascertained that these accelerate the maturation
of GFP.
We developed a new, modified GFP from these amino acids, and named it Venus, after
the planet, since it is the brightest GFP in the world. Venus is capable of emitting
fluorescence even in small amounts, because of the extremely high efficiency of
its maturation. It achieves luminescence 30-100 times that of conventional modified
GFP in Escherichia coli, and 3-100 times in mammalian cells, and is capable of
providing fluorescence that it is possible to detect even with ordinary microscopic
equipment. Accordingly, it permits more effective observation of fluorescence,
without bringing about intracellular toxicity. The amount of time from the introduction
of GFP genes until the manifestation of luminescence is also shortened from a
range of 1/2-1 day to within several hours. Brain slices that have just been prepared
may be promptly fluorescent-labeled and observed.
3. Results obtained with Venus
We confirmed that when we had labeled secretory granules present in neuroblast
lineage PC12, using Venus that we had prepared, nearly 100% of the secretory granules
were accurately labeled, with at least 10 times the luminescence achieved previously.
As a result, it became possible to observe in real time elementary processes in
secretory granules resulting from depolarization and cell stimulation. We were
also able to quantify the amount of secretion from cells by the fluorescence emitted
into the culture solution.
4. Future expectations
We expect that labeling technology using Venus will further deepen understanding
of intracellular phenomena, particularly signal transmission, and also provide
a major key to the elucidation of developmental processes, brain functions, and
the mechanisms of diseases, etc. Venus may also be expected to play a role as
a genetic reporter, faithfully reflecting the activation of targeted genes, because
of the rapidity of its maturation.
Note 1: Folding
The process whereby proteins are folded three-dimensionally from peptide chains.
Folding is necessary, first of all, in order for GFP to form chromophores, and
mature. |
|
 |
 |
Ca2+ imaging
in a Purkinje neuron of a mouse cerebellar slice.
(A) A low-magnification fluorescence photograph merged with its dark-field image.
(B) A confocal fluorescence micrograph of a Purkinje cell indicated by a box in
(A). The region monitored for Ca2+
change is boxed.
(C) Ratio images from the dendrite indicated in (B) before and after depolarization
stimulation.
Takeharu
Nagai, Keiji Ibata, Eun Sun Park, Mie Kubota, Katsuhiko Mikoshiba & Atsushi
Miyawaki
Nature Biotechnology
January 2002 Vol. 20-1, 87-90 |
|
 |
 |
|
|