In 1938 at the start of the baseball season, Lou Gehrig, a famous ball player for the New York Yankees, appeared to lose his form. The following year he was forced to retire after being diagnosed with “a disease that features the hardening of the spinal cord due to an unknown cause” called amyotrophic lateral sclerosis (ALS). Lou Gehrig died two years later at the age of 37. More than sixty years later ALS, commonly known as Lou Gehrig's disease, remains a ruthless disease with no known treatment. Victims of ALS retain clear consciousness and normal intellectual function even in the last stages of the illness when all kinetic function has been lost. Confronted with few options with regard to care, an artificial respirator is inevitable as the disease progresses.
Yet, there has been some progress in understanding ALS. One of the genes causing familial ALS, superoxide dismutase 1 (SOD1), was recently identified. Transgenic mice with the mutated human SOD1 gene were developed and present an ALS-like pathology. These mice may serve as animal models (ALS model mice) to clarify ALS's pathology and to develop suitable treatments.
ALS is characterized by motoneuron apoptosis, so ALS research has focused on the causes of cell death. However, cell death is not necessarily harmful. Throughout the development of multi-cellular organisms, certain cells are destined to die even after maturation. Cell death can be triggered to eliminate cancer cells, phagocytes and other cells harmful to, or no longer required by, the living organism; this process of apoptosis is controlled by caspase, which includes a group of proteases found widely in lower and higher orders of multicellular animals. Past experiments with the SOD1 model mouse show that caspase, a protease that executes apoptosis, is involved in neuronal death in ALS, but which caspase is involved in what mechanism nor how it might be targeted for treatment is still unknown.
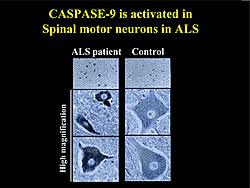
We separately introduced two kinds of apoptosis-inhibiting proteins, the human apoptosis-inhibiting protein XIAP which inhibits caspase-3, -7 and -9, and baculovirus caspase-inhibiting protein p35, which inhibits caspase -3 and -7 but not -9, into spinal motoneurons of ALS model mice. XIAP retarded the rate of paralysis following onset thus delaying death in the ALS model mice, but p35 protein did not. Therefore, we concluded that caspase-9 is inhibited by the XIAP plays an important role in the progress of the disease. Caspase-9 activation was confirmed in mouse motoneurons both biochemically and histologically. This activation has also been confirmed in human ALS motoneurons (Fig. 1).
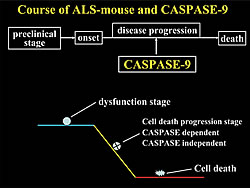
In light of these findings, inhibiting the activation of caspase- 9 in motor neurons might be a realistic target for the treatment of ALS. Furthermore, it has been recently proven that caspase-independent cell death machinery and the accumulation of abnormal proteins leading to a reduction in cell function are both involved in ALS pathology (Fig. 2). Therefore, including our results in a composite, multilateral approach seeking to inhibit these neurodegeneration pathways should be rewarding. Such a strategy should lead to the development of new and effective treatment methods for ALS in the near future.