|
 |
 |

|
Reverse Genetics in Mice
| Dr. Shigeyoshi Itohara |
| Head, Laboratory for Behavioral Genetics |
|
 |
magnified scene by clicking image
|
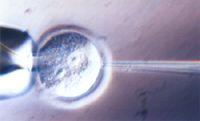
Fig. ‚P | Microinjection of DNA into the pronucleus of the fertilized mouse egg. |
|
- |
Introduction
Life science saw remarkable progress during the '90s. This progress was attained after reverse genetics technology began to be available as a realistic means of research on mammals (especially mice). In terms of the direction of gene research, genetic study used to be conducted starting from the study of phenotypes in order to search for genes, while in reverse genetics, research is actually practiced the other way round. That is, firstly a gene is identified, then a mutated form of the gene is created, and finally the phenotype is looked into.
In the beginning of the '80s it became possible to obtain transgenic mice that contained cloned genes in their chromosomes by injecting these genes into the pronucleus of fertilized eggs (Fig. 1).
|
The incredible advancements in reverse genetics in the 1990s were a result of development of technology like the homology recombination method, in which designed mutations were artificially introduced into a specific area of a cell genome. This introduction was made possible after long-term culturing of mouse embryonic stem cells (which differenciate into kinds of somatic cells including reproductive cells) in vitro was made practicable (Fig. 2, 3).
Thanks to this type of mice (so-called "knockout" mice), and thanks to reverse genetic techniques, our long dreamt aspirations of directly examining a given gene for complex vital phenomenon occurring inside an individual animal has become a reality.
The same techniques has also taken place in the field of neuroscience as well. The research group led by Dr. Susumu Tonegawa (Professor of MIT, Head of the RIKEN-MIT Neuroscience Research Center) analyzed spacial learning in mice by using this technique, revealing the impacts of this strategy in analyzing the molecular and cellular mechanisms of the complex, vital phenomenon of the brain.
Consequently, it became widely known that the mammal behavior is capable of being explained on the basis of genetic behavior. Since then, the practice of using these "knockout" mice in research has rapidly become commonplace as an essential part of studies on the developmental and information processing mechanisms in the brain.
|
|
 |
Towards the Next Generation
It is obvious that no research technique is absolutely perfect, and this holds true for this research technique as well. All of the cells in the "knockout" mice, from those in fertilized eggs to those in the adults, usually carry homogeneous mutation. Restrictions can be sometimes placed on this. For instance, some "knockout" mice which are created for the purpose of investigating the function of genes in an adult brain develop abnormally due to mutations, which lead to death prior to birth.
On the other hand, phenotype in the mutants are often difficult to confirm, even when an important function of the mutation is assumed. One of the reasons for this is that a reaction is established in which the lost function is compensated in order to maintain the homeostasis in the case of the chronic lack of a single gene. When there are many functionally overlapping genes, the potential for this increases. In addition, it is not necessarily easy to define the area or mechanism responsible for causing the abnormal behavior, because function is established on the complex neural networks. Some additional approaches are needed for this.
We are interested in analysing the molecular mechanism in which a long-term change (learning and memory) is established in the brain relating to external stimulation. In this process, we are trying to develop and improve genetical methods in mice.
At present, our methodological efforts are chiefly focused on the development of a spacio-temporal control of mutagenesis in mice. This type of technology is where an enzyme known as Cre is made to appear in certain tissues or cells so that it introduced mutation of the particular gene. The technology requires creating Cre transgenic (Tg) mice with certain cells that need to appear, as well as the proper timing of their appearance, depending on the purpose of the research.
Unfortunately, much effort and time is inevitably needed in order to find out the promoters (DNA areas from which genes emerge from) appropriate for the research subjects.
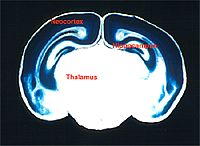
Not many promoters have been fully characterized. Figure 4 shows the results after Cre was induced in the cerebral cortex. Blue indicates the induction of the mutation into the target gene locus caused by successful expression of Cre; and the white area indicates where the target gene locus are complete and function normally.
Furthermore, if the timing of the introduction of mutation can be arbitrarily controlled, two kinds of information, that of before and after the introduction of the mutation, can be obtained from the same individual. This technology excludes different factors between individuals, and is expected to lead to more accurate evaluations of, in particular, the relationship between animal actions (e.g., learning and memory) and specific genes.
While several methodologies have been proposed today, we attempt to use a system that controls the revitalization of Cre posttranslationally, by using a steroid receptor and its ligand. In this methodology, Tg mice are created in which chimera proteins appear, and also in which Cre enzyme and the ligand uniting domain of the steroid receptor of the chimera proteins have been previously fused. Although cells having chimera proteins are specified by the Tg mice's promoter property, appearance of the cell is not enough for the recombining revitalization of Cre to be demonstrated.
However, chimera proteins are activated after the ligand has been administered from outside of the body for the purpose of to acquiring recombining revitalization. This allows for the introduction of the mutation into the target gene.
|
- |
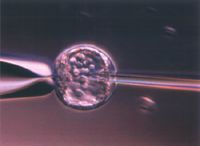
magnified scene by clicking image
Fig. 2 Microinjection of ES cells into the blastocyst of mouse.
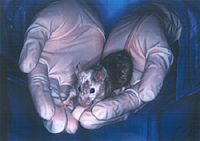
magnified scene by clicking image
Fig. 3 A chimeric mouse born from a C57BL/6-derived ES cells. Black represents C57BL/6 ES cells and white represents BALB/C cells.
magnified scene by clicking image
Fig. 4 Cerebral cortex specific knock-out system.
|
 |
Conclusion
The entire primary structure of the human genome is very close to being revealed. In order to use effectively this enormous amount of information within the field of neuroscience, reverse genetics in mice is increasingly becoming important. Needless to say, the biggest characteristic of mice which have been mutated with the reverse genetics technique is that the starting gene is specified beforehand. Meanwhile, analyzing the degree of expression of all genes, all at once, is becoming possible as genome analysis progresses (cDNA micro array method).
Four conditional settings -- pre and post mutation with the reverse genetic technique, and pre and post conditions of a certain behavior -- have come to be combined with the micro array method of genome analysis. It now appears that the field of neuroscience is witnessing an age where the behavior of animals can be studied in such a way that we are able to see both the trees (specific genes), as well as the forest (gene networks).
|
|
 |
 |
 |
|
|