Humans, like animals, connect to the world using their senses to see, hear, feel, taste or smell. Among these, we are least aware (or possibly neglectful) of our sense of smell. We may believe that this sense is less important because our social behavior is not controlled by smells, as in most animals. However, as odor usually enters our mind via the backdoor we better become attentive of it! Economically, natural and man-made odorants in perfumes and many other products in our daily life are big business, not to speak of odorants in food industries.
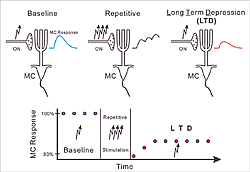
 ) before (baseline), and after (LTD), repetitive ON stimulation (
) before (baseline), and after (LTD), repetitive ON stimulation ( ). Note reduced MC responses (red trace) as compared to baseline response (blue trace) after repetitive stimulation. The graph shows the time course of the MC response strength.The graph shows the time course of the MC response strength.
). Note reduced MC responses (red trace) as compared to baseline response (blue trace) after repetitive stimulation. The graph shows the time course of the MC response strength.The graph shows the time course of the MC response strength.
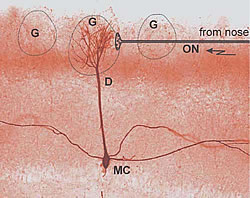
As with the other senses, olfaction uses specialized receptor cells. These are the hair cells in the olfactory epithelium that respond to particular chemicals. Activated receptor cells send impulses via the olfactory nerve (ON) to the brain. The signals are relayed in the olfactory glomeruli where they excite mitral cells (MCs) that forward odor information to higher regions of the brain (Fig.1).
Our laboratory studies the synaptic mechanism by which odor information is transmitted to the brain at ON synapses in olfactory glomeruli. Each glomerulus receives inputs from only one type out of approximately 1000 types of receptor cells, thereby relaying information about just a particular feature of odorant molecules.
Intriguingly, we discovered that ON MC synapses express a particular form of activity-dependent synaptic plasticity that we term ON long-term depression (LTD, see Fig.2). ON activation induces an excitatory postsynaptic potential in MCs. When repetitively activated (5 times per second over 20 seconds), ON MC synapses weaken and remain weakened for more than one hour. After weakening of the synapses, ON activation produces a smaller excitation of MCs. From this finding we can conclude that our brain controls the odor information that we receive from the outside world at this very first stage of olfaction. In addition, an olfactory receptor cell survives only about 60 days, and is then replaced by a new cell. Therefore, there is a life-long need to maintain and refine the specific axonal connections between the olfactory epithelium and the olfactory bulb. We hypothesize that plasticity of ON synapses is important for this process.
In a concurrent study, using elaborated electrophysiological and ion imaging techniques, we investigated the mechanism by which the ON synapses excite MCs. Activated synapses release their neurotransmitter glutamate onto the tufted apical dendrites of MCs. Glutamate activates ion channels that are formed by the glutamate receptors themselves (ionotropic glutamate receptors) but also ion channels that are indirectly controlled by so called metabotropic glutamate receptors. Both receptor types activate MCs to engage them in synchronized and oscillatory responses to odor stimuli. Synchronization of MCs that are associated with a given glomerulus (and hence a particular odor feature) likely increases the sensitivity of the odor sense. The function of the MC oscillatory responses is less clear but they may represent a temporal code (information contained in the firing pattern) that is superimposed on the frequency code (information contained in the frequency of action potentials). More than 60 years ago Edgar Douglas Adrian conducted the first pioneering studies on neuronal oscillations and synchronization in the olfactory system. Since then, the olfactory system remained an ideal place to investigate the nature of the neuronal code.