|
 |
 |
Finding
of a new cell death inducer
Motor System Neurodegeneration
Research Team |
 |
 |
Apoptosis
is a phenomenon whereby cells actively bring about their own demise, and is frequently
referred to as "cellular suicide". Although apoptosis is "death",
it is a mechanism that is indispensable to birth, development, and maintenance
of life in organisms. For example, apoptosis is needed when more cells are generated
than are required in formation of the human body. In adults, as well, impediments
to apoptosis can cause cancer and autoimmune disorders. Conversely, excessive
apoptosis apparently leads to neurodegenerative disorders such as Alzheimer's
disease.
The apoptosis mechanism is operative in insects such as flies, as well as in human
beings. The enzyme caspase plays a major role. Caspase acts by slicing through
various important proteins inside of cells, much the way scissors cut paper. It
is known as "the killer", but there also known to be proteins that halt
this killer's actions. The apoptosis-blocking protein IAP is one of these. IAP
forcefully suppresses caspase's enzymatic activity. We found that IAP suppresses
apoptosis by decomposing and scattering caspase, rather than merely deactivating
it.1 Thus, apoptosis in organisms seems to be proficiently regulated by killer
caspase, and by IAP, which acts as a bodyguard to suppress the killer.
However, surprisingly enough, proteins have been found that, in turn, block the
action of IAP. The protein known as Smac or DIABLO ordinarily remains hidden inside
mitochondria, but when a stimulus that triggers apoptosis is applied to a cell,
it emerges forcefully from the mitochondria, and bonds onto IAP, which is normally
present in the cytoplasm. IAP to which Smac has bonded is rendered incapable of
suppressing caspase, so that apoptosis can proceed.
In the present study, we found a mitochondrial protein, Omi/HtrA2, that has an
action similar to that of Smac.2 HtrA2 is released from mitochondria into the
cytoplasm in response to apoptotic stimuli (Figure 1). HtrA2 released into the
cytoplasm bonds securely onto IAP, similarly to Smac. As a result, it enhances
caspase activity, thus promoting apoptosis. However, we found that HtrA2 has an
enzymatic action that Smac lacks, whereby it severs proteins that have properties
different from those of caspase. We noted that when HtrA2 that has been rendered
incapable of bonding onto IAP is introduced into cells, its enzymatic activity
causes cells to become globular, and contract, after which they slowly die (Figure
2). This HtrA2 activity cannot be stopped by deactivating caspase. Hence, we found
that HtrA2 is a completely new kind of cell death inducer, with a double action
whereby it both enhances the action of caspase by impeding that of IAP, thus promoting
apoptosis, and at the same time brings about cell death through an enzymatic activity
of its own, regardless of caspase.
We found that when the expression level of HtrA2 is reduced by means of special
methods, cell death occurred less readily, indicating that it plays an important
physiological role. In other results that we have obtained recently, we found
that the release of HtrA2 from mitochondria coincides with the timing with which
symptoms of some kinds of neurodegenerative disorders proceed, and this aroused
our interest as to its relationship to these diseases. We are making efforts at
present to identify factors that regulate HtrA2. We hope that this research will
provide the basis for development of new therapies for neurodegenerative diseases. |
1. Suzuki, Y., Nakabayashi, Y., and Takahashi, R.: Ubiquitin-protein ligase activity
of X-linked inhibitor of apoptosis protein promotes proteasomal degradation of
caspase-3 and enhances its antiapoptotic effect in Fas-induced cell death. Proc.
Natl. Acad. Sci. U.S.A. 98: 8662-8667 (2001)
2. Suzuki, Y., Imai, Y. Nakayama, H., Takahashi, K Takio, K., and Takahashi, R.:
A serine protease, HtrA2, is released from the mitochondria and interacts with
XIAP, inducing cell death. Mol. Cell 8: 613-21 (2001)
| |
 |
 |
| Fig.
1 |
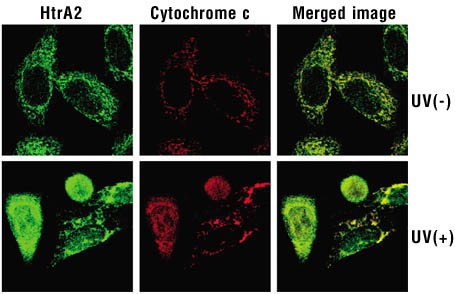
Here it can be seen that when cell death is induced under ultraviolet (UV) radiation,
HtrA2 is released from the mitochondria (which have a reticulated appearance)
into the cytoplasm. Known mitochondrial cell death inducer cytochrome-c exhibits
exactly the same behavior. |
| |
 |
 |
| Fig.
2 |
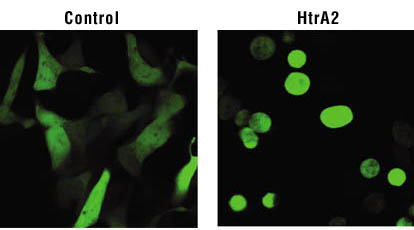
When HtrA2 is made to express through gene transfer, the cells become globular,
shrink, and die. In this case, caspase activity is not increased, and cell death
cannot be suppressed by caspase inhibition. |
|
 |
 |
|
|