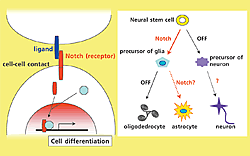
Notch is a single-pass transmembrane receptor, which is transactivated by membrane-bound ligand proteins through direct cell-cell contact. It has been shown that activation of Notch signaling in neural stem cells induces astrocyte differentiation at the expense of neurons.
Background
Circuits formed by neurons in the brain enable function by exchanging electrical signals between projections, or axons, that extend out from those neurons. Neurons are abundant in the brain, but for each of those neurons there are at least ten glial cells. These cells coat the neurons providing structural and functional support, facilitating interneuronal communication, and supplying nutrition for the neuron it coats. During brain development, a neural stem cell can become either glial or neuronal cells. Mutually interactive processes produce a complex network of neurons and glia by controlling the processes of cell differentiation and growth in the brain.
Recent research confirmed the role of the Notch signal in the differentiation of neural stem cells into neurons or glia. Activating Notch signal, in vivo or in vitro, in neural stem cells reduces the number of neurons produced and increases the number of astrocytes produced. Glia are one type of astrocyte. When the Notch signal is not activated the number of neurons increases. Hence, the Notch signal promotes astrocyte production from neural stem cells (Fig.1). However, Notch signal is essential for the development of all types of tissue as embryos that lack the genes for the Notch signal die at the start of development. Hence, the role of the Notch signal with regard to the formation of neural circuits remains unknown.
Research Techniques and Results
We discovered the gene DNER before investigating Notch signaling. DNER is expressed exclusively in differentiated brain neurons and the membrane protein the gene encodes. Our analysis of the amino acid sequence of DNER revealed characteristics similar to a ligand protein that activates the Notch signal. So we determined the interaction between DNER and Notch using culture cells. When cells expressing DNER encountered those expressing Notch, a molecular binding occurred between them. This activated the Notch signal. Clearly, DNER can act as a ligand for the Notch signal.
In fact, DNER and Notch are expressed in brain cells during circuit formation, and the two appear to interact closely during this period. Cerebellar Purkinje cells, neurons in which DNER is primarily expressed, are coated by Bergman glial cells expressing Notch (Fig.2). Without Bergman glia, Purkinje cells cannot function normally. Therefore, we used premature Bergman glia taken from infant and young mice to study the physiological significance of DNER and the Notch signal in these two cells. The glia were cultured with Purkinje cells with or without DNER and the morphological effects were studied. We observed extensive Bergman glia activity in the presence of DNER. When Notch signaling was chemically, or through a mutation, inhibited, this DNER effect disappeared.
In the cerebella of knockout mice with reduced DNER expression, Bergman glia failed to project to the neurons and neural circuit development was noticeably delayed (Fig.2). Therefore, brain-specific DNER-Notch signal promotes glial differentiation essential for proper neuronal function.
Future expectations
DNER is strongly expressed in the neurons of many vertebrates, including fish and other animals, while neural circuits are being formed. The gene also regulates the differentiation of a wide range of neural cells, not just those in the cerebellum. And, it seems, DNER contributes to the development of the complex neural circuitry seen in vertebrates. Regenerative medicine tends to focus on neuronal loss by generating newly differentiated neurons from neural stem cells in vitro and transplanting them into the brain. Yet, these new neurons need to integrate into the existing circuitry to restore the normal brain function. Many types of cells are required for this to happen. The control factors of neural cell differentiation presented here are likely to be a valuable in developing effective regenerative medical treatments for the brain.





![Fig. 2: [left] DNER expression in Purkinje cells (magenta) surrounded by Bergmann glial fibers (green) in the mouse cerebellum. [right] Bergmann glia in wildtype and DNER knockout mice. Bergmann glial fibers are deformed in the absence of DNER.](../files/research0202-thumb.jpg)
