Dendritic filopodia are long, thin, headless, and actin-rich protrusions abundantly found in immature neurons. They are flexible and highly motile structures that facilitate the formation of physical contacts between nascent dendrites and axons. As development proceeds, filopodia are morphologically and functionally transformed into mature spines that constitute a post-synaptic structure of more than 90% excitatory synapses in adult mammalian brain. Thus, dendritic filopodia is an important structure regarded as the precursor of spine, but little is known about the molecular mechanisms underlying the formation of dendritic filopodia and the transition from filopodia to spines.
Telencephalin (TLCN; ICAM-5) is a cell adhesion molecule belonging to the ICAM family of the immunoglobulin superfamily. TLCN is expressed exclusively in spiny neurons within the most rostral brain segment, telencephalon, which takes charge of higher brain functions such as learning, memory, emotion, consciousness, integration of sensory information, and control of voluntary movements. In the telencephalic neurons, TLCN is specifically localized to dendrites but not to axons. Ontogenic appearance of TLCN parallels the timing of dendritic outgrowth, spine formation, and synaptogenesis in early postnatal life and the expression persists into adulthood.
A dendrite of cultured hippocampal neuron at 14 days in vitro double-immunostained for TLCN (green) and MAP-2 (magenta; a marker for dendritic shafts). TLCN is abundantly found in long and thin protrusions called dendritic filopodia.
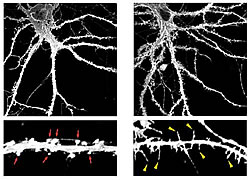
Low-power (upper panels) and high-power (lower panels) images of control (left) and TLCN-overexpressing (right) hippocampal neurons at 22 days in vitro visualized by expression of membrane-targeted GFP. TLCN overexpression caused a marked increase in number of dendritic filopodia.
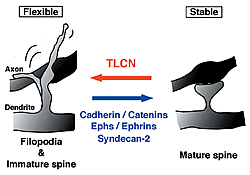
Unlike other cell recognition molecules that facilitate spine maturation and synapse stabilization, TLCN maintains highly dynamic and plastic structure, dendritic filopodia, and slows spine maturation.
In this study, we reported that TLCN is a negative regulator of spine maturation. Using cultured hippocampal neurons, we examined detailed localization and function of TLCN in spine development and synaptogenesis. At early stages of development, TLCN was abundantly present in dendritic filopodia (Fig. 1). At later stages, TLCN tended to be excluded from mature spine synapses in which postsynaptic PSD-95 clusters were apposed to presynaptic synaptophysin clusters. To elucidate the function of TLCN in spine maturation, we analyzed the dendritic morphology of TLCN-overexpressing neurons and TLCN-deficient neurons. Overexpression of TLCN resulted in a dramatic increase in the density of filopodia and a concomitant decrease in the density of spines (Fig. 2). Conversely, TLCN-deficient mice showed a decreased density of filopodia and an acceleration of spine maturation in vitro as well as in vivo. These results demonstrate that TLCN normally slows spine maturation by promoting the filopodia formation and negatively regulating the filopodia-to-spine transition (Fig. 3). In addition, we found that spine heads of mature neurons are wider in TLCN-deficient mice compared with wild-type mice. Thus, the preservation of immature synapse by TLCN may be an essential step for refinement of functional neural circuits also in the adult telencephalon.