Background
The neural circuit in the cerebral cortex is mainly composed of excitatory neurons that use glutamate as the transmitter and of inhibitory neurons that use gamma-aminobutyric acid (GABA) as the transmitter. It is known that the visual cortex contains orientation-selective neurons that respond only to those visual stimuli that are in a specific orientation (inclination). This characteristic supports the concept that the brain executes feature-selective information processing. However, the mechanism that produces the feature selectivity is still unknown.
We attempted simultaneous observation of the responses to light stimuli of the excitatory and inhibitory neurons in the visual cortex using functional two-photon excitation imaging. This method utilizes the phenomenon by which a molecule is excited when absorbing two photons simultaneously and the excitation wavelength is twice that of one-photon excitation. Since light with a longer wavelength can reach deeper parts of living tissue, the two-photon excitation imaging method was developed in 1990 as a method for observing fluorescence in the deeper parts of biological samples and it has since been used to observe the morphology of neurons and similar structures.
The functional two-photon excitation imaging method was developed more recently for use in the observation of a large number of neurons using the Ca2+ fluorescence indicator, the fluorescence intensity of which varies according to the activities of neurons. This is a revolutionary method that is capable of the simultaneous observation of hundreds to thousands of neuron activities, which significantly exceeds the previous limit using the microelectrode method, which was tens of neuron activities at most. However, it still does not allow observation of the excitatory and inhibitory neurons separately.
Results of Research
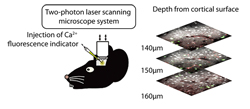
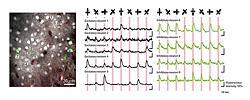
We attempted to dye the excitatory and inhibitory neurons separately by applying functional two-photon excitation imaging method to transgenic mice with which only the inhibitory neurons express the green fluorescent protein. The traditional Ca2+ fluorescence indicator has a problem of interference with green fluorescence but we succeeded in avoiding such interference by injecting a different Ca2+ fluorescence indicator and also a dye to differentiate the glia cells. This newly developed technique allowed us to dye the component parts of the cortical neural network of living mice - the excitatory neurons, inhibitory neurons and glia cells - differently, and to observe their activities simultaneously (Fig. 1).
When we applied visual stimuli to anesthetized mice with light from various orientations by using this technique, we found that the excitatory neurons present significant orientation selectivity and that the inhibitory neurons respond almost uniformly to stimuli regardless of orientation (Fig. 2). This finding suggests that inhibitory neurons control the expression of the orientation selectivity of excitatory neurons while at the same time inhibiting their overall response.
Significance of the Research
In the cerebral cortex, a large number of neurons form a 3-dimensional neural network. Previously, the function of this network could only be estimated from the data obtained by observing the activities of one or a few neurons with the use of microelectrodes. The present research has overcome the limitations of the electrode method and has succeeded in coloring a large number of cells in the neural network according to their types, and of enabling observations of their activities along the time axis. This new method presents the potential for elucidating the very complicated mechanisms of information processing of the cortical neural network. The new method is also expected to be applicable to the future development of artificial circuitry with equivalent abilities to that of the cortical neural circuitry.





