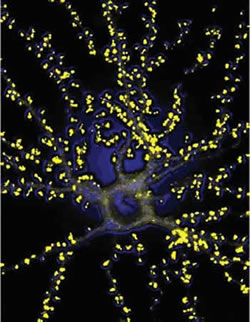
In the brain, billions of neurons touch and communicate with each other via specialized structures called synapses. Synapses are the nodes and junctions of the huge neuronal network that comprises the nervous system. Throughout development, and even in adult life, the brain responds to experience by changing the strength of communication through individual synapses. In addition, new synapses can be constructed and existing synapses can be eliminated in response to experience, thereby changing the spatial pattern of connections between neurons. In this way, long-term information can be stored by the nervous system in the form of altered structure and chemistry of synapses. This so-called “plasticity” of synapses and networks is believed to be the basis of learning and memory in the brain.
Because of their key role in information processing and storage, it is important to elucidate the structural design of synapses. If we can understand the molecular architecture of synapses, it should help us to understand how synapses perform their signaling functions and how they can adapt dynamically to environmental influences. By using biochemical, genetic and imaging approaches to study the structure and dynamics of neuronal synapses, we seek to understand the fundamental mechanisms of brain plasticity.
Our initial efforts were focused on identifying the building blocks (protein components) of the synapse. As a result of many years effort by our laboratory and other laboratories, a catalog of synaptic proteins has been compiled which is nearing completion. We believe that several hundred different protein “building blocks” are needed in total to construct a synapse. Despite their identification, the precise function of most of these synaptic proteins is unknown. The next stage is to decipher the functional roles played by these molecular components in synaptic signaling and plasticity and how they are important for the animal s behavior. With better understanding of synaptic structure and function, we hope to identify possible targets for drugs and treatments that could help to alleviate mental illness and neurological disease such as Alzheimer’ s dementia in humans.
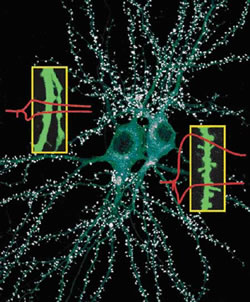
A current focus of research in our lab is the specialized structure of the synapse called “dendritic spine”. Dendritic spines are tiny compartments that protrude from the dendrites of neurons, and they “receive” the inputs of most excitatory synapses in the brain. Dendritic spines grow during normal maturation of the brain but are lost or abnormal in shape in many human neurological diseases, including mental retardation and dementia. Moreover, dendritic spines are mobile, and they change their number and shape in response to the animal’s experience and in response to electrical signaling in the brain. For instance, rats raised in rich environments have more spines than those that live in unstimulating environments. Stimulation of synapses can lead to the production of new spines. Therefore dendritic spines are believed to be critical morphological structures of synapses. It is widely held that the regulation of dendritic spine number, size and shape is of central importance in the plasticity of synapses and learning and memory.
Our goal is to understand the molecular mechanisms that control the growth and morphology of dendritic spines. At present, little is known about the genes and proteins that regulate the formation, size/shape and dynamic motility of dendritic spines. Moreover, the actual role of spines in brain information processing or cognitive function in vivo (e.g. learning and memory) is not established. By identifying specific protein components of dendritic spines, we have opened in-roads into the molecular mechanisms that underlie spine formation and growth.
We have identified specific proteins that promote the growth of dendritic spines (e.g. Shank, cortactin, SPAR) and proteins that induce the loss of dendritic spines (e.g. Rap, Homer1a, SNK protein kinase). The level and activity of these proteins are also regulated in the brain, so we expect that these proteins play a role in the normal processes of development and plasticity of dendritic spines.
One specific example of a spine regulatory protein (named “Shank”) serves to illustrate our research. We discovered Shank as a large protein that is highly concentrated in synapses. It binds to several other synaptic proteins and assembles them into a complex, and therefore we call Shank a “scaffold” protein. When Shank level is increased in neurons in culture, dendritic spines grow bigger and synaptic strength is increased. When Shank level is suppressed in cultured neurons, dendritic spines are lost and synaptic strength is weakened. Normally, Shank level is low at birth and increases during the first few weeks of postnatal brain development in mice and rats, coinciding with the period of spine formation and growth. We believe that Shank promotes the morphological and functional maturation of dendritic spines and their associated synapses.
What happens when Shank is defective in vivo? To answer this question, we created mutant mice that lack one of the genes that encode Shank. These mutant mice look outwardly normal. Histological examination of brain neurons revealed that they had smaller synapses and dendritic spines, as expected from previous in vitro studies. Upon behavioral testing, however, we found something unexpected - Shank-deficient mice seem to learn faster than normal mice. This is one of the few examples of a mutant mouse that actually performs better in learning than normal animals. Thus in formal genetic terms, Shank seems to be a suppressor of learning.
An interesting aspect of brain plasticity and learning and memory is that it declines with age. Young animals (including humans) can learn more quickly than mature animals. For instance, a child can learn foreign languages and new motor skills more quickly and more completely than an adult. What is the explanation for this developmental change in brain plasticity? We presume that the composition of the brain and its synapses is changing in some way during maturation. Perhaps the Shank protein, which increases with postnatal brain development, contributes to the decline in plasticity and adaptability that accompanies the maturation of the brain. We are pursuing further experiments to test this idea.