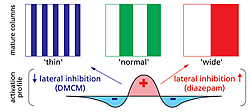
Columnar architecture is the fundamental unit of neocortical organization across mammalian species. Morphological clusters of thalamic axon terminals serving the right or left eye, for instance, tile the primary visual cortex into alternating ‘ocular dominance’ domains. Early sensory experience during a critical period of development is widely believed to individualize these columnar maps. What determines their unique layout and periodicity, however, remains unknown. In self-organizing computational models, it is the recipient cortical circuits that set the final spacing of columns. Sensory input is spread locally by excitation but limited at a distance by farther-reaching inhibition (Fig. 1), establishing which ‘neurons that fire together wire together’
By adjusting the profile of intracortical activation during development, lateral inhibition in particular can produce narrow or wide columns in silico. We have validated these long-standing theoretical predictions in vivo through the direct infusion of benzodiazepines in kitten visual cortex. Such drugs come in three varieties, including agonists like diazepam (valium), inverse agonists such as the β-carbolines (e.g. DMCM) and antagonists that block the actions of both. All are known to act on particular GABAA receptor subtypes with opposite effect on chloride flux. Enhancing inhibition with benzodiazepine agonists throughout the critical period produced a thirty percent increase in column width, while inverse agonists yielded column shrinkage (Fig. 1).
As local imbalances in neuronal activity influence segregation, column formation is unlikely to be explained purely by genetic instruction. Bidirectional control of columnar architecture is unprecedented and simulated in computer models when long-range (rather than local) inhibition is preferentially altered.
Specific GABA Circuits for Plasticity
Indeed, not all GABAergic neurons are likely to be involved. Monocular occlusion during the critical period exaggerates segregation, eventually yielding expansion of open eye columns at the expense of deprived-eye axons, which become reduced in size and complexity. We previously demonstrated that such competition requires GABAergic function in vivo, as plasticity lies dormant in mice lacking the GABA-synthetic enzyme GAD65 (Hensch et al, Science 1998). A single class of interneuron with the potential for controlling long-range inhibition and synchrony in visual cortex may provide the scaffolding upon which sensory input is discriminated.
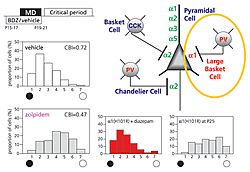
Although widely heterogeneous, GABA cells in the neocortex are remarkably precise in their connectivity (Fig. 2, right). Parvalbumin (PV)-positive contacts include axon-ensheathing Chandelier cells and soma-targeting large basket cells. The latter extend a wide-reaching, horizontal axonal plexus, which can span ocular dominance columns in the cat. Individual GABAA receptor α-subunits are trafficked to discrete post-synaptic sites on the pyramidal cell axon, soma and dendrites (Fig. 2, right). For example, α2 subunits are preferentially enriched at the axon initial segment and at basket cell synapses innervated by cholecystokinin (CCK)-positive axon terminals (Klausberger et al, J Neurosci. 2002). Importantly, the α-subunits determine benzodiazepine binding through a single amino acid residue in their N-terminus. ‘Knock-in’ of a point mutation at this site renders individual GABAA receptor subtypes insensitive to benzodiazepines in separate lines of mice (Rudolph et al, TIPS 2001).
Weak inhibition within visual cortex early in life (like GAD65 deletion) prevents experience-dependent plasticity (Fagiolini & Hensch, Nature 2000). Loss of response to an eye deprived of vision can be initiated prematurely by enhancing GABA-mediated transmission with zolpidem, a GABAA α1, 2, 3-selective benzodiazepine agonist (Fig. 2, left). Systematic analysis of the mouse ‘knock-in’ mutation further demonstrated that only one of these subtypes controls the critical period. The α1-containing circuits were found to drive cortical plasticity (Fig. 2, bottom), whereas α2-enriched connections separately regulated neuronal firing.
This dissociation carries implications not only for models of brain development, but also the safe design of benzodiazepines for use in human infants. Pharmaceutical development of α2-selective ligands to treat hyperexcitability (epilepsy) would avoid the rapid, premature sensitivity to sensory input through α1-containing GABAA receptors. These receive PV- (but not CCK-) positive, large basket cell synapses upon the soma (Fig. 2, right), consistent with our anatomical results implicating long-range inhibition to sculpt cortical architecture.
Critical periods, in general, are a process of selecting the best neural representation of the world from among many competing inputs that bombard the maturing nervous system. Growth and function of specific lateral inhibitory connections offers a rational, cellular substrate to gain broader insight into experience-dependent brain development across regions, its disorders, recovery from injury and improved life-long learning.