Molecules in living cells move constantly. The type of motility determines whether this movement is diffusion or transport-driven. Fluctuation, or small-scale movements, and migration, or large-scale movements, also distinguish bio-molocular behavior. In addition the roles of time and space on regulating the effects of molecular movements on cell proliferation and division have been uncovered by using fluorescent proteins, such as Green Fluorescent Protein (GFP), taken from coral or sea anemone, that label molecules within living cells. The molecules can then be observed under a microscope through fluorescent imaging. However, this technology can only reveal the steady-state distribution of labeled molecules. Quantitative information on movement cannot be gathered.
Fluorescent fading technology has successfully tracked slow molecular movement. However, as fading occurs slowly, this technique is not suitable for rapid movement. It was not until 2002 that instantaneous fluorescent switching became possible by using light to trigger a switch using Kaede. Kaede is a fluorescent protein that can change from green to red when exposed to ultra-violet light. Unfortunately photo-labeling with Kaede is permanent. This limits the number of labeling operations and biomolecular movements that can be observed. Molecular changes are constant, occurring before and after every stimulus, thus limiting Kaede's usefulness. Sustained observing of biomolecular movements in a single cell requires developing a technology capable of periodic, fast rebooting that primes the subject for the next observation via photolabeling.
We cloned a new green fluorescent protein from stony coral (Pectiniidea) and genetically produced a mutant with photochromic properties, that is one in which light absorption produces reversible color, in its monomeric state. This mutant will emit a bright green fluorescence even exposed to a weak blue light. However, a very strong blue light reduces blue absorption band and instead shows absorption in the violet domain. Exposure to weak violent light restores the protein's original characteristics. Therefore, its green fluorescence can be switched on and off by exposure to two different wavelengths. We dubbed this protein Dronpa after the the Japanese word Dron, which is a ninja term referring to instant disappearance of the body, and pa, short for photo-activation.
Using a laser diode, letters can be written, read and erase using an argon laser. Scale bar: 300µm.
To explore the possibilities of Dronpa, we spread the protein over a glass cover and used an ordinary confocal laser-scanning microscope equipped with an argon laser (488nm) and laser diode (405nm) to highlight, read, and erase the green fluorescence (Fig.1). Dronpa worked like a rewritable molecular memory system that might be similar to a CD-RW or DVD-RW disc. The photochromic molecules currently attracting attention for possible photo-optical applications are typically organic compounds such as diarylethene. Our success in generating a protein that induced photo-chromic fluorescence is novel and promising. Dronpa is likely to be incorporated into many applications that exploit it bio-molecular properties, including genetically manipulated expression. Uses within aquatic environments and that take advantage of its biodegradation potential are also plausible.
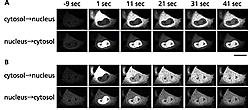
We used reversible photo-labeling technology based on “Dronpa” for our cellular biological experiments. Within a cell many molecules move between the cytoplasm and nucleus in response to external stimuli. Among these is the enzyme MAP kinase (MAPK), which, when subjected to growth factor stimulus, will migrate from the cytoplasm to the nucleus and be phosphorylated by transfer factors to control gene expression.
(B) The same cell 10 minutes after the EGF stimulus.
In addition to its important roles in cell growth and division, MAPK is also actively involved in various diseases. We produced ERK1-Dronpa, by tagging the MAPK isoform ERK1 (Extracellular signal-Regulated Kinase 1) with Dronpa in cultured cells. Overall fluorescence in the cells was erased and then photo-labeling ERK1-Dronpa was introduced into the cytoplasm. Migration into the nucleus was observed. Overall fluorescence was erased again and introduced photo-labeled ERK1-Dronpa into the nucleus and observed migration to the cytoplasm. The migrations for both were of low efficiency (Fig. 2A). Next, epithelial growth factors (EGF) were spray on the same cell and migrations to and from the cytoplasm and nucleus were observed. Translocation of ERK1-Dronpa started to accelerate in both directions 10 to 15 minutes following EGF application (Fig. 2B). We demonstrated for the first time that MAPK information control in the nucleus is controlled by the speed at which this enzyme shuttles between the cytoplasm and nucleus.