Introduction
Awareness of scents occurs as odor molecules are processed by the olfactory system. This system first detects the information through odorant receptors in the nose, relays it to relevant areas of the brain, and then create the odor image of objects. Since the 1991 discovery of the odorant receptor gene family by Linda Buck and Richard Axel, for which they earned a Nobel Prize in 2004, research on the biological basis of smell has made significant advances. By the use of multi-disciplinary techniques from molecular biology, genetic engineering, electrophysiology, and functional imaging, various studies have revealed the expression patterns and functions of odorant receptors in the nose and identified a corresponding odor map in the olfactory bulb.
The Laboratory for Neurobiology of Synapse at RIKEN BSI is investigating the mechanisms of formation, maintenance, functional expression and plasticity in the neural network of the olfactory system. Their work is exhaustive, covering both the molecular and cellular activities in the neural network and behavioral processes using mice and zebrafish. What follows is a brief summary of three recent findings about the formation of the olfactory neural circuitry made by the lab.
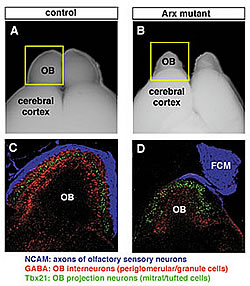
(A, B): The olfactory bulb of Arx-deficient mice is much smaller than that of the normal mice.
(C, D): Triple-immunofluorescence labeling of olfactory bulb sections. The interneurons in Arx-deficient mouse (red) do not enter into the olfactory bulb and abnormalities are seen also in the mitral cell layer (green). Most olfactory sensory cell axons (blue) remain outside the olfactory bulb leading to the formation of an abnormal structure, fibrocellular mass (FCM).
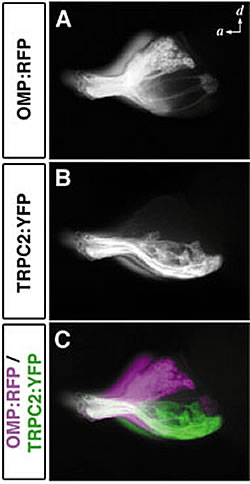
Axons of ciliated and microvillous olfactory sensory neurons were labeled with (A) RFP and (B) YFP by the use of OMP and TRPC2 gene promoters, respectively. (C) Mutually exclusive glomerular innervation by the two types of olfactory sensory neurons. Ciliated sensory neurons (magenta) project axons to dorsal and medial regions, while microvillous sensory neuron (green) send axons to the lateral region of the olfactory bulb.
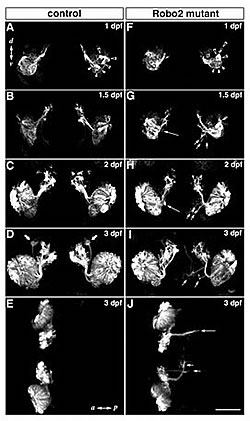
In Robo2 mutant zebrafish (Right), many olfactory axons cannot reach the olfactory bulb and misroute into other brain regions (arrow).
(A-D, F-I): Frontal views
(E. J): Dorsal views
dpf: days of postfertilization
Arx: a transcription factor essential for development of the mouse olfactory system
In the olfactory system, two types of neurons undergo continuous turnover even in adulthood: olfactory sensory neurons in the olfactory epithelium and inhibitory interneurons in the olfactory bulb. Arx is a homeobox-containing transcription factor expressed in the interneurons from an early stage in embryonic development until maturity. Lab member Sei-ichi Yoshihara conducted detailed analyses of Arx's role in developing olfactory neural circuitry using Arx knock-out mice. Kunio Kitamura then working at the Mitsubishi Kagaku Institute of Life Sciences, now at the National Center of Neurology and Psychiatry, also contributed to this project. They found that interneurons of Arx-deficient mice could not move to their target locations in the olfactory bulb and the olfactory bulb of Arx-deficient mice was subsequently smaller than that in normal mice (Fig. 1). Arx-deficient mice also showed abnormal axon projections from the olfactory epithelium to the olfactory bulb. These results clearly demonstrate the importance of Arx in the development of olfactory neural circuitry and that hypoplasia, or olfactory bulb underdevelopment, may cause abnormalities in the establishment of those neural connections between nose and brain. Moreover, they suggest that Arx might regulate the transcription of a putative signaling molecule that guides olfactory axon projection from the epithelium to the bulb.
Visualization of two distinct neural pathways in the zebrafish olfactory system
Zebrafish develop rapidly and possess simpler neural circuitry that is similar to mammals. Therefore, using advanced genetic engineering, these animals are ideal for studies of developmental neurobiology. In addition, because embryos are transparent, it is easy to observe normal and mutant development in vivo, and in real-time. Cell migration, axonal and dendritic extension, synapse and neural circuit formation can be monitored by expressing various fluorescent proteins in specific neurons. For the olfactory system, the numbers of odorant receptor genes and of olfactory bulb glomeruli in zebrafish are smaller than those in mice by an order of magnitude. Hence, researchers investigating the reception, coding, processing and plasticity of odor information can easily exploit the benefits of this animal model.
In zebrafish, there are two types of olfactory sensory neurons in a single olfactory epithelium: one with cilia and one with microvillus. Yuki Sato isolated those genes that express specifically in either of these sensory neurons (OMP, CNGA2, and OR-type odorant receptors in ciliated sensory neurons; TRPC2 and V2R-type receptors in microvillous sensory neurons). She established transgenic fish lines expressing fluorescent proteins with different spectra under the control of OMP and TRPC2 promoters. As a result, she found that the two types of olfactory sensory neurons innervate glomeruli in different regions of the olfactory bulb in a mutually exclusive manner (Fig. 2). This suggests that, like mice, the olfactory system of zebrafish has two distinct neural circuits with each handling different types of olfactory information for different functions (i.e. pheromones and odorants).
Robo2: an axon guidance molecule controlling odor map formation in the olfactory bulb
Finally, Nobuhiko Miyasaka identified the molecule that mediates axon projection of olfactory sensory neurons using transgenic zebrafish. He found that Robo2, a gene expressed in the olfactory sensory neurons only during the initial stage of development, is an indispensable guidance molecule for axons heading toward the olfactory bulb (Fig. 3). He also discovered that Robo2 mutant zebrafish, even into adulthood, have abnormal neural connections between nose and brain. These mutants cannot develop a normal 'odor map'. Clearly Robo2 is indispensable to the formation of those initial neural connections between the nose and the brain. We believe that the proper formation of initial axon scaffold at the earliest stage of development is important for establishment of the precise glomerular map in adult.
Conclusion
Smell is one of the most primitive senses. It has been passed down, or up, from unicellular organisms during over a billion years of evolution to homo sapiens. And, while the sense of smell may not be considered as essential in humans as it is in mice or fish, imagine how empty life would be without coffee's aroma to lure us from our beds or the tantalizing temptations of steaks roasting on the barbeque on hot summer evenings...