Cognition is a balance between internal motivations and external stimulation. Take a paradigmatic example: visual attention. It is thought that volitional shifts of attention result from "top-down" signals from the prefrontal cortex while the automatic capture of attention by salient stimuli results from "bottom-up" signals in visual cortex. But there is little direct evidence for this, partly because we lack data on the precise timing of neural activity between brain areas that can provide insight into how signals flow between them.
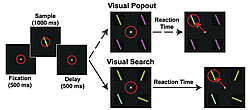
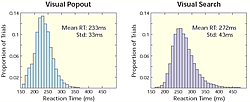
So, we have been conducting a series of experiments using multiple electrodes implanted in two brain areas known to be critical for shifting attention: the prefrontal (lateral prefrontal cortex or LPFC and the frontal eye fields or FEF) and parietal cortex (the lateral intraparietal area, or LIP, Fig.1) while monkeys engage in both bottom-up and top-down attention tasks. Top-down and bottom-up attention were contrasted using visual search and pop-out tasks (Fig.2). Both tasks required the monkey to find a target stimulus in an array of distractors. They differed in how the distractors related to the target. In the pop-out condition, all of the distractors are identical and differ from the target along a single dimension (either color or bar orientation) and attention is automatically drawn to the contrasting stimulus. In the visual search, each distractor independently differs from the target along either dimension. Because the target matches some of the distractors in each dimension, monkeys must actively search the display for the target, requiring top-down control. Our monkeys showed a shallower increase in reaction time with increasing distractor number for pop-out over search. For neurophysiological experiments, we used one target and three distractors; reaction times for visual search were both higher on average and more variable than during the pop-out condition (Fig.3). These are hallmarks of automatic pop-out versus active search.
To determine when neurons in each area found the target, we calculated the amount of information that each neuron carried about its location as a function of time during the trial. So far, approximately 1/3 of all randomly recorded neurons in each area showed selectivity for the target location during either the pop-out or visual search task.
The data thus far shows that in the pop-out condition, there were clear differences in timing between areas: LIP found the target first, followed by the LPFC and then the FEF (180 ms, 120 ms, and 50 ms before saccade, respectively). In the search condition, the results were quite different. Neurons began finding the target just before the saccade (about 50 ms before) and in the reverse order: the frontal cortex was first (the LPFC and FEF about 55 ms before the saccade), followed by LIP. In fact, although LIP neurons found the target first and well before the saccade during bottom-up attention, in top-down LIP neurons did not begin to significantly reflect target location as a population until after saccade. Taken together, these data suggest two modes of operation: in pop-out, a bottom-up, fast target selection that occurs first in LIP, while in search, a top-down, longer latency target selection is reflected first in frontal cortex. To our knowledge, these are the first direct demonstration that these areas may have different contributions to these different modes of attention.
Synchrony between different anatomical areas has been suggested to help dynamically bind regions together to aid their communication and has been shown to play a role in attention within a cortical area. To investigate the role of synchrony in top-down and bottom-up control of visual attention, we measured the degree of coherence between electrodes in LIP and the frontal cortex. Synchronous activity was measured between the local field potential (LFP) using all possible pairs of simultaneously recorded, selective electrodes.
Across both attention tasks, there was an increase in synchrony between LIP and frontal cortex is observed for a middle and upper frequency band. However, the degree of increase each band depended on whether attention was top-down or bottom-up. There was a greater increase in synchrony in the middle-frequency band (refined to 22 -34 Hz) than during pop-out. By contrast, during pop-out, synchrony between LIP and frontal cortex was significantly higher in the upper frequency band (35 - 55 Hz) than in search.
Thus far, these results suggest that competition within posterior cortex (e.g., LIP) appears to automatically resolve the target location during pop-out, while visual search relies on a biasing signal directing attention originating in the frontal cortex (Fig.1). The pattern of selectivity found in LIP is consistent with the area carrying, in part, a 'saliency map' capable of automatically selecting the highest saliency target, but failing to find a target that does not have an outstanding saliency value. As the animal shifts between the two behaviors, so must the relationship between parietal and frontal cortex in order to reflect the current task. Synchrony is one possible mechanism for easily regulating the strength of connections between areas. Differences in the frequency bands of synchrony between the top-down and bottom-up condition suggest that the brain takes advantage of synchrony at different levels to help regulate information flow.