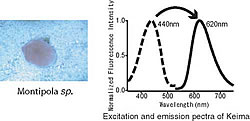
Our laboratory develops different fluorescent proteins. Using coral from the Ryukyu Sea, we extracted a fluorescent protein with a maximum excitation wavelength of 440nm and a maximum fluorescence wavelength of 620nm. The large difference between these wavelength peaks, called a Stokes shift, reminded us of the movement of the knight in chess. So, we named it after the knight in Japanese chess, "Keima"
Molecules labeled with fluorescent protein markers will generate fluorescence signals when exposed to certain wavelengths. We can observe these signal changes over time using fluorescence correlation spectrometry (FCS) and fluorescence cross-correlation spectrometry (FCCS) was developed from FCS. Since FCCS analyzes the fluctuations between two colors, it is possible to monitor the interaction between two molecules. Two fluorescent dyes that are excited simultaneously are required for FCCS analysis. Traditionally, simultaneous excitation is using two lasers simultaneously to capture the signals. However, this method is complicated because it is difficult to align the foci of two lasers to the same location in a small domain and the two fluorescence signals often interfere. Fluorescence resonance energy transfer (FRET) can also impact the quality of observation. If Keima, with its large Stokes shift, can be used with a cyan fluorescence protein (CFP) with a small Stokes shift, these problems may be eliminated.
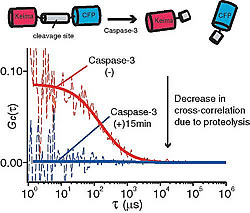
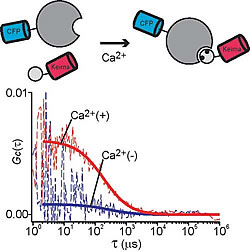
We paired Keima-CFP and performed two FCCS analyses. In the first, we monitored the peptide proteolysis of Caspase-3 over time (Fig.2-a). For the second, we monitored the calcium-dependent interaction between CaM and CaMKI: Although CaMKI is a relatively large protein the interaction could be observed with high reproducibility. Keima and CFP coupling did not affect monitoring efficiency (Fig.2-b). The effects of molecular size and coupling are important in determining the direction of molecular interaction.
Only one laser is needed if we use Keima, thereby simplifying the FCCS technique. As a result, prices of FCCS systems should fall and the technique will be more widely used. We also believe that efforts that use Keima to discover the lead compounds for the development of novel drugs will be significantly more efficient.
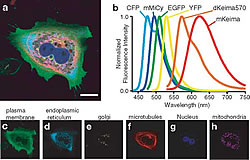
It is increasingly more important to untangle complex phenomenon by observing multiple factors simultaneously. We successfully performed a six-color imaging study of a sub-cellular organelle using Keima and other fluorescent molecules that were excited with a 458 nm argon laser (Fig.3).