1. Background
In the field of cell biology, fluorescent proteins, such as GFP (Green Fluorescent Protein) that is found in the medusa varieties of jellyfish, are used to label living cells. A cell will become fluorescent by the simply introduction of these proteins because they are capable of independently forming chromophore a chemical group that, when exposed to light, can produce colors. However, while helpful in marking a group of cells, these protein markers cannot label an individual cell among a group of cells.
Cells that originate from stem cells that can reproduce and undergo division, will differentiate, migrate and execute various functions depending on its environmental conditions. To analyze these processes, it is necessary to target one cell and mark it at a specific time to be able to trace its development, but the techniques currently available, including introducing GFP as a virus vector, are unsuitable because they rely heavily on probability and have insufficient temporal and spatial accuracy in marking cell targets. Neurons form complex networks throughout the central nervous system by projecting their axons towards other cells. To analyze these networks, it is necessary to trace the contours of individual neurons; yet, with standard optical observation methods, this is impossible because neuronal networks are intricately woven. The conventional method to grasp the overall picture of a neuron is to insert a glass electrode into a selected neuron and inject it with a fluorochrome, such as lucifer yellow, but this process is difficult. An easier, noninvasive technique is long overdue.
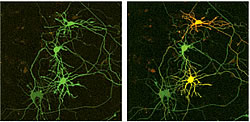
(A) Image before marking.
(B) One day after transfection with the cDNA of Kaede, the top and bottom neurons (indicated by an arrow and arrowhead) were illuminated in their cell bodies using a violet laser diode (405 nm) for 0.5 and 0.25 sec, respectively. Scale bar, 50µm.
2. Kaede: a new fluorescent protein
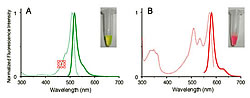
To obtain cnidarian animals that emit fluorescence that could be used to clone fluorescent protein genes with new characteristics, our research team scoured the coral reefs near the islands of Okinawa and the aquarium shops in downtown Tokyo. Trachyphyllia geoffroyi (Figure 1) is a stony coral that emits colorful fluorescence in green, yellow and red. The cloned fluorescent protein obtained from this coral initially emits a bright green fluorescence (excitation maximum: 508 nm, fluorescence maximum: 518 nm, Figure 2A). However, when a sample of this protein happened to be left on a laboratory bench near a window, we discovered that the fluorescent light changed to red overnight (excitation maximum: 572 nm, fluorescence maximum: 582 nm, Figure 2B). We found that the protein exhibited a wavelength conversion that responded to UV rays (photoconversion). This fluorescent protein was named "Kaede" (which means maple tree) because its color changes from green to red much like the maple trees in autumn. Kaede is as bright and stable red as it is when green. This photo-conversion is easily achieved by exposing the protein to UV rays from a regular Xenon lamp for a short time, and the excitation light used to observe the red and green fluorescence does not produce photo-conversion. These properties suggest that Kaede should become a particularly useful marking technique for cells.
3. Research results using Kaede
In an experiment using HeLa cells, Kaede in red would diffuse rapidly throughout the cell (diffusion coefficient: 29 um2/sec) when, following continuous illumination by UV light, the intensity ratio of red to green fluorescence increased 2,000 times. This demonstrates the fluorescent protein's effectiveness for marking cells. Illuminating a small part of a selected cell by UV light for a brief time is sufficient to cause the color of the whole cell to change to red. Figure 3A shows neurons developing Kaede labeled in green in a high-density nerve culture system with axons and dendrites intricately interconnected. After illuminating the cell body of a specific neuron with a ultra-violet laser (405 nm) for different time periods, the targeted cell became red, or yellow, even at its projecting points (Figure 3B). The adjacent neuron tangled remained green; the bonded joint between these two neurons is clearly visible.
4.Future expectations
By using light, this cell marking technique is highly effective for analyzing how individual cells extend or migrate within a group of tangled, or changing, cells in a three-dimensional environment. Furthermore, because it only requires a standard light microscope, this marking technique is likely to be widely used in the future. A detailed analysis of other physiological phenomena is possible by generating transgenic animals in which Kaede is present throughout the body and using the latest photoirradiation techniques, including lasers, to visualize changes in development. This approach expands the available array of live imaging techniques that use fluorescent proteins and will become an invaluable tool for researchers working to understand development processes, brain functions, and disease mechanisms. To help improve current photo-conversion techniques, the molecular mechanisms of photo-conversion are now being investigated using Kaede.