Why do we sleep? Although we spend roughly a third of our lives engaged in this activity, its purpose remains largely a mystery. An obvious thought might be "to rest." While this is true for most of the muscles in our body, it is hardly the case for the brain. With the first measurements of brain waves, the electroencephalogram (EEG) revealed a highly dynamic pattern of activation during sleep. These come in two varieties: large amplitude, slow-wave and waking-like, desynchronized oscillations. The latter were found just fifty years ago to be accompanied by rapid eye movements and so is referred to as REM sleep (Aserinsky & Kleitman, 1953), ushering in the modern era of sleep physiology.
During the course of a night, a stereotypical bout of first slow-wave then REM sleep constitutes a 90 minute cycle in humans. Intriguingly, the balance of time spent in either phase gradually shifts from mostly slow-wave at sleep onset to largely REM by morning. It is generally believed that REM is associated with dreaming, but we know that dreams of a different sort also occur during slow-wave sleep. Psychologists, philosophers and neuroscientists alike have long wondered why the brain bothers to be so active when it is cut off from the outside world. An attractive idea is that sleep could be an off-line mode, when the brain actively reorganizes the events of the day in preparation for the next.
Synaptic plasticity has thus been proposed to be intimately related with sleep (for review see Miyamoto & Hensch, 2003). Speculation spans the extremes from 'sleep consolidates memories' to 'sleep clears away useless connections' all in an effort to allow the brain to do what it does best, learn. From birds singing, rats running mazes, to humans playing tetris, numerous reports have recently identified instances of neuronal replay during sleep of activity patterns seen during learning. Moreover, intervening sleep sessions (even as short as a nap) often improve performance thereafter. Deprive a subject of sleep and plasticity is slowed down. Thus, it certainly is possible to 'learn in your sleep,' as well as to find inspiration.
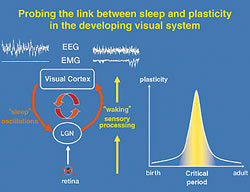
Our work aims at a cellular link between the two brain functions of sleep and plasticity, using the developing visual pathway as a model system. Thalamo-cortical circuits process sensory input during waking and spontaneously generate the slow-wave oscillations characteristic of sleep (Fig. 1). This shared neural substrate is then an ideal site to probe direct interactions, especially during well-known critical periods of development. For instance, appropriate maturation and maintenance of visual function requires normal sensory experience. Depriving one eye of vision during a critical period in early life leads to a rapid shift of responsiveness in favor of the open input in primary visual cortex, which is further enhanced by sleep. Our results clearly show that it is a two-way relationship: not only does sleep help the brain to learn, but sleep itself is changed through experience.
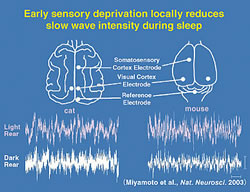
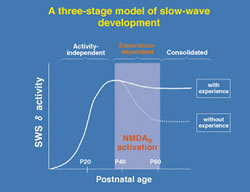
It may seem surprising at first, but in fact the very EEG rhythms that define the various sleep stages are not fixed. Heavy use or complete disuse of sensory input can locally increase or decrease the size of the slow-waves seen in the corresponding brain regions. We have found that the nature of sleep is changed by visual experience (Miyamoto et al. 2003). Raising cats or mice in complete darkness from birth (dark-rearing: DR) produces a reversible reduction of slow-wave delta (1-4 Hz) EEG activity in primary visual cortex but not in other brain regions of the same individuals (Fig. 2). Notably, the amount of sleep is unaffected. Gene-targeting in mice further reveals slow-wave changes to be NMDA receptor-dependent. Interestingly, the susceptibility of sleep to sensory deprivation is itself restricted to a late critical period for developmental plasticity (Fig. 3).
If EEG rhythms are a reflection of dream content, then weaker slow-waves in one part of the brain versus another may lead to a change in sleep quality. Clinical diagnoses of sleep disorders based on EEG analysis must in the future consider the past experiences of each individual (especially during critical developmental periods) in addition to more acute, global changes in brain chemistry.