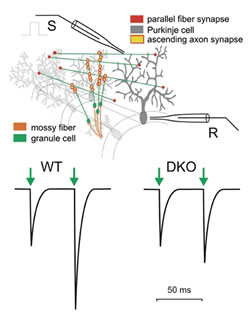
The neuronal circuitry of the cerebellar cortex represents a simple basic circuit diagram with a few major cell types organized in a massive parallel design. The relative simplicity of the basic circuitry contrasts with an impressive number of building elements. For instance, cerebellar granule cells are the most numerous group of nerve cells in the mammalian brain. The axons of granule cells ascend from the granular layer to the molecular layer where they form synapses with Purkinje cells. The axons then bifurcate and give rise to the parallel fibers that run in the frontal plane where they form additional en passant synapses with Purkinje cells. In the rodent brain, there are ~10'000'000'000 granule cells forming ~175'000 synaptic contacts with each Purkinje cell. This apparent simplicity has encouraged theoreticians and experimentalists for decades in their hope to unravel the neuronal operations done by the cerebellar cortex. Theoretical work suggested that the numerous parallel fiber-Purkinje cell synapses constitute a memory device, an idea that received support from experimentalists describing long-term plasticity at these synapses. Theoretical and experimental work led also to the idea that the small conduction velocity of parallel fibers and associated delays between granule cell and Purkinje cell activity are critical for cerebellar function. The latter hypothesis addresses neuronal processing that might, at least conceptually, be separated from long-lasting neuronal memory. Several mutant mouse strains are known in which long-term synaptic plasticity (long-term depression, LTD) correlates with motor defects, but experimental data showing altered cerebellar neuronal processing with unaltered synaptic long-term plasticity are sparser for the cerebellum. We were, therefore, enthusiastic when, in collaboration with Dr. Rolf Joho (UT Southwestern, Dallas, USA), we identified mice that were ataxic following genetic deletion of two potassium channels, Kv3.1 and Kv3.3, that are expressed at high levels in cerebellar granule cells. We hypothesized that the observed motor deficit (ataxia) in these potassium channel-deficient mice might be caused by altered neurotransmission at the granule cell-Purkinje synapse. If this were the case, investigating these mice would shed light on the neuronal processing that takes place in the cerebellum.
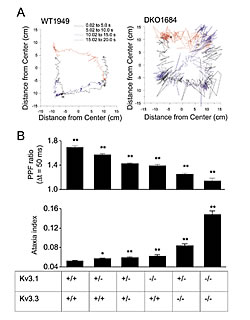
B) Short-term plasticity (PPF ratio) and ataxia indices obtained from mice lacking varying numbers of functional Kv3.1 and Kv3.3 alleles.
Using patch-clamp recordings from Purkinje cells, we investigated short-term plasticity at parallel fiber-Purkinje cell synapses in normal and potassium channel-deficient mice. In normal mice, an action potential in parallel fibers induces transmitter release at a synaptic contact with a Purkinje cell only at a low probability, such that only every 10th or 20th action potential induces a response in the Purkinje cell. However, when stimulating a bundle of parallel fibers, a sufficient number of synapses are activated so that a robust compound excitatory postsynaptic current (EPSC) can be measured in the Purkinje cell. When a second action potential quickly follows, the probability of neurotransmitter release is increased and a larger EPSC is observed. This phenomenon, called paired-pulse facilitation, may be quantified as the ratio of the second to the first synaptic current (paired-pulse facilitation ratio or PPF ratio). We found that the PPF ratio was much smaller in double-mutant (DKO) mice that lack both Kv3.1 and Kv3.3 (Fig. 1). Since the size of the second EPSC depends on the presence of a preceding EPSC, PPF is a form of short-term plasticity.
We then went on and investigated mice carrying different numbers of nonfunctional Kv3.1 or Kv3.3 alleles. Motor performance was quantified using an ataxia index determined with a force-plate actometer and compared to the corresponding PFF ratio. We found that both measures, ataxia index and PFF ratio, varied in a gene dosage dependent manner (Fig. 2).These data indicate a correlation between short-term plasticity at the parallel fiber-Purkinje cell synapse and motor performance. There remain, however two important issues. First, the above data are only correlative and do not demonstrate a causal relation between changes of short-term plasticity and motor performance. To address this issue, mice in which Kv3 channel function is reintroduced in specific neuronal cell types are currently generated in Dr. Joho's laboratory. A causal relationship between the alterations at the parallel fiber-Purkinje cell synapse and motor performance would be indicated if reintroduction of these channels in granule cells improved motor performance. The second issue is related to the classical debate about the importance of granule cell-Purkinje cell synaptic transmission along the full (anatomical) extent of the parallel fibers. Researchers have argued that the majority of parallel fiber synapses fail to activate Purkinje cells and that Purkinje cells are mainly driven by synapses formed by the ascending branch of the granule cell axon (see Fig. 1), or that only parallel fibers close to the point of bifurcation form functional synapses. It has been argued that many parallel fiber synapses are non-functional (silent synapses) or that strong inhibitory mechanisms limit their efficacy. We used voltage-sensitive dye and intrinsic fluorescence signals to address the question of whether granule cells excite Purkinje cells only locally via the ascending branches of their axons or, more widespread, along the entire parallel fiber trajectory. White matter stimulation of the mossy fibers elicited a beam-like fluorescence change along the trajectory of parallel fibers. Simultaneous imaging and extracellular recording demonstrated the association between the beam-like fluorescence signal and Purkinje cell spiking. These data support the notion that parallel fiber activity, evoked either locally or through the mossy fiber-granule cell pathway, can activate postsynaptic Purkinje cells along more than 3 mm of the parallel fiber trajectory.