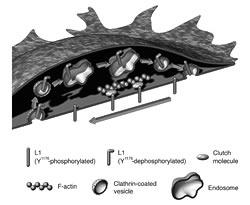
L1's movement towards the back of the growth cone occurs via retrograde actin flow. At the back of the growth cone, L1 is dephosphorylated at tyrosine (Y1176) in the cytoplasmic domain and internalized via the clathrin-dependent pathway to be transported forward by vesicular transport and re-inserted into the plasma membrane at the head of the growth cone's plasma membrane. Recycled L1 then reponds extracellular signals to initiate traction forces that contribute to the forward migration of the growth cone (image11)). Animation of this movement can be viewed at http://www.brain.riken.jp/en/h_kamiguchi.html ~Download movie file [PowerPoint w/mov].
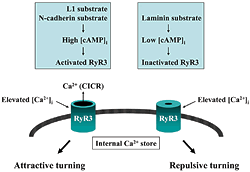
The extracellular environment of the growth cone (L1, N-cadherin, laminin, etc.) controls RyR3 activity by regulating intracellular cAMP concentrations [cAMP]i. Axon guidance molecules elevate the cytoplasmic calcium ion concentration [Ca2+]i in a localized area of the growth cone. This Ca2+ signal triggers a secondary Ca2+ release from the intracellular store (Ca2+-induced Ca2+ release, CICR), depending on the RyR3 activity. When Ca2+ signals are accompanied by CICR generated on only one side of the growth cone, the cone turns toward the signals (attractive turning). When Ca2+ signals are not accompanied by CICR, a repulsion of the growth cone is observed. RyR3 is a key molecule for determining the direction of axon elongation.
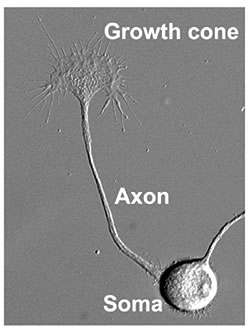
How does neuronal circuitry develop? To understand circuit formation, our lab is studying the molecular mechanisms of neurite formation that connect a single neuron to others. In general, the neurites differentiate into axons, which transmit electrical stimuli to target cells in remote regions, or dendrites. The cell body, or soma, of the neuron is between ten and twenty micrometers in size, whereas an axon may elongate over distances that can extend more than one meter. In this way, a neuron can be tens of thousands of times longer than its soma, depending on the final destination of its axon. How that axon traverses such terrain and correctly identifies its target are riddles that may be solved by studying the axonal growth cone (Fig. 1).
In 1890, after conducting fixation studies on spinal cord samples, Santiago Ramó y Cajal described growth cones as a " concentration of protoplasm of conical form, endowed with amoeboid movements" 1). 17 years later Ross Harrison successfully observed the kinetics of growth cone movement2). Only recently, was the forward motion of the growth cone mechanism identified: axons are pulled along by the growth cone3). The growth cone guides the elongating axon towards its correct destination using molecular signals from the extracellular environment and pulling the axonal projections along the proper path4). In this way, roles of a diverse cast of molecular players in neuritic function (axon elongation and axon guidance) are clarified, it will be possible to understand the mechanisms of axonal network formation.
Cell Adhesion Molecules
In 1949, Bickers and Adams characterised X-linked hydrocephalus(XLH)5), a congenital disorder with enlarged cerebral ventricles and axonal malformations. A surgical procedure can be performed at birth that inserts a ventriculoperitoneal shunt to drain the cerebral fluid from the brain can alleviate some of the symptoms, but functional prognosis remains poor. The incomplete neural development that is seen in XLH most likely stems from abnormal neuronal differentiation (impaired migration and circuit development)6). As more than 60% of XLH patients have a mutation in the L1 cell adhesion molecule, this gene is a likely causative factor. Therefore the role of L1 in neural development is studied. Several years ago, our laboratory showed the biophysical mechanisms underlying how L1 stimulates axon elongation: L1 in the plasma membrane of the growth cone transmits the traction force generated by the actin cytoskeleton (to be described later) to the extracellular environment, and axon elongation is driven by a caterpillar-like motion of L1 on the growth cone surface and inside of the cell7)(Fig. 2).
Cytoskeleton
There are two major components of the cytoskeleton in the growth cone: microtubules and actin filaments. The microtubules are tracks on which molecular motors transport vesicles (membranous organelles). Actin filaments play a central role in cellular morphogenesis, locomotion, and migration. Space-specific polymerization/depolymerization and myosin motors move actin filaments backward in the growth cone. This retrograde actin flow interacts with the cytoplasmic domain of cell adhesin molecules via clutch molecules. Traction generated by the retrograde actin flow is thereby transmitted to the extra-cellular environment. Our laboratory recently identified a clutch molecule involved in neurite formation. We also suggested that mutations in the L1 cytoplasmic domain that contribute to the production of XLH may cause axonal hypoplasia by hindering the clutch connection8). The spatiotemporal mechanisms that control clutch engagement/disengagement are an important factor in determining the speed and direction of axon elongation.
Cell membranes
Many functional molecules in the plasma membrane of growth cones are transported via microtubules. Recycling of cell adhesion molecules toward the leading edge of the growth cone (Fig. 2) occurs through endocytosis and vesicular transport. Axonal membrane transport regulates the surface area of the growth cone and functional molecule expression locally. A recent study suggested that left/right asymmetry of membrane transport in the growth cone may participate in direction changes associated with axon elongation.
Proteins and lipids in the cell membrane form micro-domains (e.g. lipid rafts) in which specific functional molecules are concentrated. Our laboratory developed a technique that selectively inactivates lipid rafts in local regions within a cell. With this method we discovered that lipid rafts at the leading edge of the growth cone are essential for axon elongation9). Lipid rafts have also been implicated in spatial control of L1 recycling in the growth cone. We have started a comprehensive analyses of the intracellular signal transduction system by lipid rafts to elucidate the molecular mechanisms underlying axon elongation.
Intracellular signals
Many axon guidance molecules induce directional changes by increasing concentrations in cytoplasmic Ca2+ in the growth cone. We analyzed the relationship between the unilateral Ca2+ signal in the growth cone and its turning response. Our findings are shown in Fig. 310). Upon interaction with the extra-cellular environment, cell adhesion molecules conrol intracellular cAMP concentration and protein kinase A activity in the growth cone. Activated protein kinase A up-regulates the type-3 ryanodine receptor (Ca2+channel in the intracellular store) that determines the direction of axon elongation. In this way, type 3 ryanodine receptor integrates independent signals generated by axon-guiding and cell adhesion molecules, to correctly navigate the growth cone towards it destination.
Conclusion
Growth cone migration is controlled by
1. cytoskeletal dynamics,
2. efficient clutch engagement, and
3. recycling of cell adhesion molecules.
Our laboratory is studying the spatiotemporal control of these three factors to elucidate the molecular mechanisms of axon elongation and guidance. We are also analyzing the functions of the axonal tip in damaged adult central nervous tissue that might identify those mechanisms that hinder axon regeneration within damaged tissue.