Researching the activity and function of inhibitory neurons is as important as that for excitatory neurons. In our laboratory, we want to clarify one of the primary neurotransmitters of inhibitory neurons: GABA (Gamma-Aminobutyric Acid). Roughly 30% of neurons are GABAergic inhibitory neurons, which contain glutamic acid decarboxylase (GAD) a GABA-synthesizing enzyme. In addition, these neurons also have a vesicular GABA transporter (VGAT) in the membrane. Combined, these molecules synthesize GABA at the presynaptic site, pack GABA into vesicles, and maintain sufficient levels of by transporting excess GABA out of the cell or breaking it down.
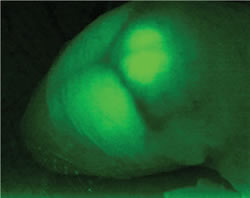
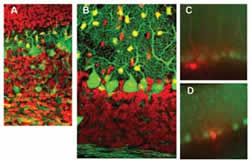
GABAergic neurons and neurites (including Purkinje and intermediate neurons) are in green. Intermediate neurons migrate from the white matter (lower) to the molecular layer (upper). Red indicates granule cells.
Sample taken from 20 day old mouse cerebellum. After taking recordings of electrical activity in Lugaro (C) and candelabrum cells (D) of the intermediate neurons, we stained those cells red. GABAergic neurons, including large Purkinje cells arranged in an array and small astrocytic cells, are green.
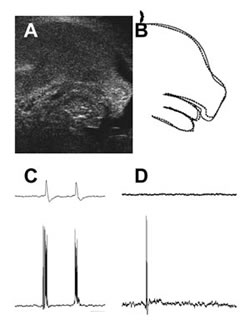
(A) Ultrasound image of the 14-day mouse fetus. (B) Diagram, dotted line shows the shape when the mouse opens its mouth. A normal mouse fetus will open the mouth sometimes, but GAD67 embryo does not.
(C) and (D) show the respiratory electrical activities recorded from the brain stem of a mouse fetus (18th day). With the normal mouse (C), neurons fire with respiration (the lower trace) and motor nerves act synchronously (upper trace). Neurons in GAD65 and GAD67 knockouts (D), only fire occasionally (lower trace) and motor nerves do not work (upper trace).
In a collaborative project involving several BSI and non-BSI laboratories, we prepared several strains of genetically altered mice to investigate the roles of GABA. In our lab, we monitored abnormalities caused by GABA deficiencies in GAD65, GAD67, and VGAT knockout mice. We also inserted GFP, green fluorescent protein gene into the GAD67 gene in order study GABAergic neurons in vivo (Fig. 1). As the cerebral cortex develops, excitatory and GABAergic inhibitory neurons are produced in different locations and migration patterns. We analyzed the movement of GABAergic neurons together with the Murakami Lab at Osaka University.
The unique structure of the cerebellum is the product of two processes. While excitatory granule cells are produced at the surface and then migrate into deeper regions, Purkinje cells and inhibitory interneurons (all GABAergic) migrate to the surface from the ventricular side. With our GFP stained mice, we showed that intermediate neurons proliferate in the white matter and migrated toward the cortex like Purkinje and granule cells (Fig. 2A, B).
In electrophysiological studies of GFP mice with Yale University, we showed that GABAergic neurons affect hormone secretion regulation in the hypothalamus and internal sodium regulation by the circumventricular organs with researchers at the Japanese National Institute for Basic Biology. We are presently analyzing the mechanism that regulates GABAergic transmission in the cerebellar cortex by adrenaline and are studying those intermediate neurons that, for various reasons, have not been well researched including Lugaro and candelabrum cells (Fig. 2C, D).
GAD65 knockout mice have 50% less GABA than normal mice and they present epileptic-like seizures and seemingly abnormal expressions of anxiety and fear. Since GABA is mainly synthesized from GAD67 during fetal development, GAD67 knockout mice have less than 10% of GABA in normal mice at birth and compromised neural activity. In addition, the spontaneous respiratory discharge and limb movements often seen in normal fetuses are not observed in knockout mice (Fig. 3). Since these mice die immediately after birth from complications associated with abnormal palate formations, we cannot study these anomalies further using these animals.
Through crossbreeding, we also generated mice with no GAD65, GAD67, and VGAT. As expected, these animals displayed no GABA activity, but they also showed no rhythmic activity in motor neurons in the brain stem and spinal cord (Fig. 3D). Also, the inhibition of motoneuron regulation led to an excessive number of neurons following development. Yet, with such a large number of phenomena and mechanisms associated with GABA function not yet understood, our research is really just skimming the surface. There is a lot of work yet to do.
Although GABA was believed to be specific to brain function in animals, GAD and GABA can also be found in plants and microorganisms. In fact, GABA was recently found to have an important role in the pollination of plant pistils and to protect them from UV hazards. In animals, researchers have found GABA in cells of the pancreas, hair root, and reproductive organs , but the function of GABA in these areas needs to be clarified.
A single substance can, and often does, serve many different roles in a organism based on where it finds itself; GABA is no different.. And organisms are able to produce what it needs, and remove what is not needed, to survive. We get enough GABA in our regular diet from foods like potatoes and tomatoes. And when GABA is not needed by the body, it is broken down by the liver without ever entering the blood stream. Even when GABA is absorbed in human intestines, it is immediately decomposed in the liver, etc. so that almost no GABA enters the blood. So scarping down on all those GABA enhanced food products probably will not enhance brain function.