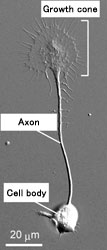
All known functions of the brain rely on the formation of accurate neural circuits distributed all over the body. During development of the neural circuit, individual axons extend toward target cells or organs at distant locations. The longest axons in the human body have to navigate more than a meter to connect with the right targets. At the distal end of an elongating axon, there is an ameba-like enlargement called the "growth cone" (Fig. 1). A growth cone is guided along its correct route by various "guidance cues" that functions like road signs. These guidance cues attract or repel the growth cones, and are known to increase the cytoplasmic calcium ion concentration inside the growth cones. However, the molecular machinery downstream of the calcium signals remains to be found.
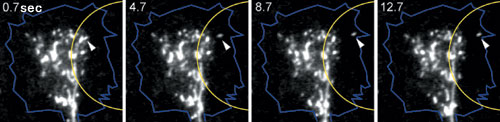
When an axon sprouts from the spherical neural cell body, the plasma membrane covering the surface of the growing axon must also expand concurrently. Exocytosis, the process by which an intracellular vesicle fuses with the plasma membrane, occurs frequently in growth cones. To examine vesicle movement in the growth cone, we labeled membrane vesicles inside neural cells derived from embryonic chick spinal cords, with a lipophilic fluorescent dye called FM1-43. When calcium signals were artificially generated on one side of the growth cone to induce attractive turning, membrane vesicles move towards the calcium signals (the side facing the new direction) (Fig. 2). Using a pH-sensitive fluorescent protein developed by the Laboratory for Cell Function Dynamics, RIKEN BSI (Head, Dr. Atsushi Miyawaki), we then verified whether these membrane vesicles undergo exocytosis. We found that the vesicles translocated towards the calcium signals did fuse with the growth cone plasma membrane. Then, with the use of tetanus toxin, which inhibited exocytosis, we confirmed that attractive growth cone turning induced by the calcium signals required exocytosis. These experiments demonstrate that asymmetric vesicle transport and subsequent exocytosis in the growth cone is essential for attractive growth cone turning.
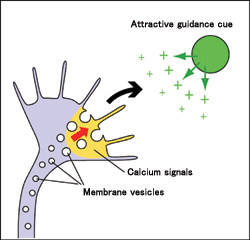
We have successfully shown that growth cone turning is driven by a simple mechanism in which the necessary building blocks are selectively supplied to the side of the growth cone facing the new direction (Fig. 3). This discovery not only provides a new concept for neural circuit development, but also contributes to regenerative medicine that may help repair the injured spinal cord and other neural degenerative diseases.
(Takuro Tojima)