Background
Neurons and glia are the primary cells of the central nervous system, and the majority of glia cells are astrocytes that protect neurons from their surroundings. Recent studies also suggest that astrocytes might be involved in synapse formationthe process by which neurons differentiate, mature, and make connections with each other. In fact, when astrocytes are mixed with cultured neurons the formation of synapses is more efficient than in cultured neurons alone. Yet, we still do not know how the astrocytes interact with neurons to form synapses.
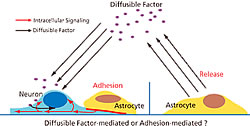
Two factors are likely to be involved in this process (Fig. 1). A “diffusible factor” that would promote synapse formation has been actively investigated using astrocyte culture medium. This search has been facilitated by simple “sprinkling experiment” which gradually mixes the potential diffusible substance with cultured neurons. Cholesterol, which is secreted by astrocytes, was identified as an attractive candidate in the manner. However, research into an “adhesion factor” that allows astrocytes to adhere to the membranes of neurons to promote synapse formation has been limited. This is because the experiment process is laborious, requiring the researcher to carefully monitor astrocyte behavior over a period of several hours as they slowly entangle isolated neurons. The Laboratory for Cell Function Dynamics has developed a technology for imaging the forms and functions of cells and neurons that simplifies this experimental procedure to analyze how neurons and astrocytes adhere.
Research Techniques and Results
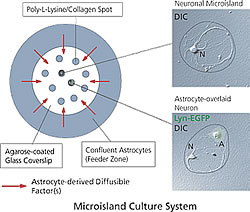
We individually cultured undifferentiated neurons from the rat hippocampus and constructed a system to analyze the effects of astrocyte adhesion (Fig. 2). A slide cover glass was coated with agarose and placed on an array of poly-L-Lysine and collagen islands. Each island houses an individual neuron. Astrocytes were also cultured at edge of the culture dish to saturate the culture medium with the diffusible factor secreted.
The number of neurons is strictly controlled in this construction, as only one neuron is grown on each island. In this setup, the diffusible factor is positive (+) and the adhesion factor is negative (-) in the culture dish. In a second set-up, neurons are cultured with astrocytes that are place directly over them. Here, the conditions change: both the diffusible and adhesion factors are positive (+). We compared synapse formation in the two conditions and analyzed the roles of both the diffusible and adhesion factors of the glia cells in neuron maturation.
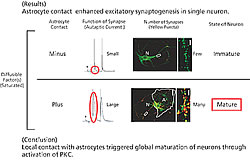
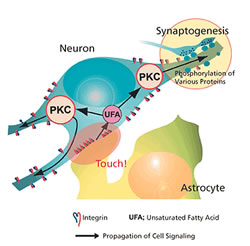
We discovered that neurons with astrocyte adhesion showed 5-6 times more noticeable synapse formation than those having a negative adhesion factor (Fig. 3). Therefore, astrocyte-derived adhesion factor is required for complete synapse formation. We also found that integrin, a protein present on the neuron, is involved in this adhesion. Two days of imaging showed that astrocyte adhesion is confined to a certain area of the neuron. So we investigated the molecular mechanism underlying the process from local adhesion to the overall synapse formation and neuronal maturation. Protein kinase C, which is activated by the unsaturated fatty acid, was found to function adequately (Fig. 4).
Future Expectations
Researchers in regenerative medicine are investigating ways to treat neuronal diseases by transplanting neural stem cells. For this to be successful, a complete understanding of neuronal development is required. As a neuron is “mature” only after it has formed synapses with surrounding neurons and is communicating with them, so knowing how to induce synapse formation and trigger cell maturation in undifferentiated neural stem cells will be important. Our research into the effects of astrocyte adhesion in neuronal maturation and the mechanisms of intracellular signal transduction caused by this adhesion. We hope that these findings might establish the basic guidelines for the brain treatment research in the future.