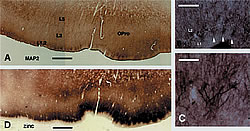
B: Adjacent section reacted by zinc histochemistry. Note the patches of zinc positive terminations, similar in size and location to the dendritic patches.
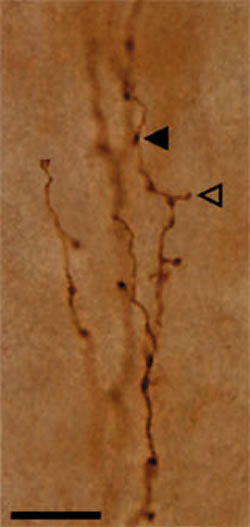
C and D: progressively higher magnification of the dendritic clusters. Three of these are shown by white arrowheads in B. Scale bar = 1.0mm for A,D; 160µm for B; 80µm for C
Our lab investigates cortical organization with a view to structural substrates of visual perception and other higher order processes. Basic features of cortical structure have been known for over 100 years: cortex has layers (as opposed to a nuclear collection of cells). It has multiple distinguishable areas. Since the 1940’s, some kind of ver tical columnar organization has been recognized as well; but how these and other features relate to cortical functioning remains very much a core question. One view, particularly popular around 1990, proposed that cortex is fundamentally “uniform,” and consists of a very large array of repetitive circuits serving a basic cortical computation. The implied simplicity is attractive and elegant; but it can be argued that no concrete blueprint has yet emerged of either a truly canonical circuit or of a universal cortical process. Indeed, many examples can be given of area- and species-specific neuron types, architectures, and genes or combinations of genes; and current opinion now commonly assumes an immense neural flexibility ? and, by inference, diversity ? as more compatible with a rapidly changing and unpredictable environment.
The connectional organization of cortical areas has been established by many labs that used tracer injections.Injections were usually extracellular, where hundreds or eventhousands of neurons were included within injection sites that typically measured 0.5-3.0mm in diameter. These techniques showed what fields were interconnected, and could also indicate neurons of origin or layers of termination. On this basis, two large classes of cortical connections were distinguished: feedforward and feedback. In a convenient simplification, these are sometimes equated with ”bottom-up” and “top-down” cognitive phenomena; but the detailed functions, subtypes, and even the basic circuitry of these connections remain largely unknown.
Our lab has so far concentrated on presynaptic inputs, such as corticocortical, thalamocortical, and more recently amgydalocortical. These are all excitatory and use glutamate as neurotransmitter; but future work will need to address how inputs from different sources converge and interact in relation to particular postsynaptic targets. Anatomical investigations of these questions require reliable visualization of both preand postsynaptic components, and we are currently working on ways to achieve this.
A major new result from this lab has been the finding of a honeycomb-like small scale modularity in the upper cortical layers of many areas in several species (Ichinohe et al, 2003; Ichinohe and Rockland, 2004). With some area-specific variation, this structure consists of a subpopulation of zinc-enriched corticocortical terminations (in the honeycomb walls), intermingling with inhibitory parvalbumin-positive terminations, and interdigitating with thalamocortical terminations. Thalamocortical terminations are glutamatergic, but do not use zinc as co-factor. Different apical dendritic subpopulations are preferentially situated within the honeycomb walls (dendrites of layer 2 neurons) or hollows (dendrites of deeper neurons). Since this structure is preferentially localized to the border of layers 1 and 2, we postulate that it is closely associated with cortical feedback and amygdalocortical inputs, which terminate densely in this zone. In fact, work in progress corroborates that neurons in layers 2 and 6, the primary source of feedback cortical connections, are a major source of the zinc positive terminations.
One implication of these findings is that in neocortex, as in limbic cortices, layer 2 has a distinct and prominent role. In addition, the high levels of zinc in the honeycomb walls recall the mossy fiber terminations in the CA3 sector of the hippocampus, which have long been associated with synaptic plasticity. At the synaptic level, zinc is known to bind to and inhibit NMDA receptors as well as other postsynaptic molecules. Thus, the subpopulation of zinc positive glutamatergic synapses potentially adds yet another dimension to modulation of synaptic dynamics and plasticity. Finally, it is important to emphasize that the zinc honeycomb shows area variation and, in primates, is more developed in motor- and limbic-related (as op posed to sensory) sectors. We are hoping to investigate what this might mean for structural and functional specializations at the cellular and circuitry level.
Our interest in zinc-enriched terminations has been from the perspective of normal cortical organization. However, synaptically released zinc is believed to play a significant role in pathological conditions: epilepsy (protectiveinaction?) and Alzheimer’s disease (pathogenic in action?).
Some investigators have compared the supposed actions of zinc as an inter- and intracellular messenger to those of calcium. Regardless of this possibility, it is already clear that zinc plays an important role in cortical function; and the zinc honeycomb offers an interesting new means of probing synaptic modulation and circuit specializations.