Alzheimer’s disease is caused by an excessive accumulation of amyloid-b (Ab) peptide in the brain. In most individuals Ab accumulation begins between 40 and 80 years of age and the rate of accumulation is accelerated with age. Consequently, about half of the persons aged 85 or older suffer from Alzheimer’s disease or mild cognitive impairment that is commonly considered a transition state of the disease. Acetylcholine levels are lower in patients with Alzheimer’s disease. Therefore, drugs such as Donepezill to block acetylcholinesterase activity, which breaks down the neurotransmitter acetylcholine, are currently used to treat Alzheimer’s disease. However, this is a symptomatic therapy that cannot prevent the progress of the disease. We identified neprilysin as an Ab-degrading enzyme and clarified that the deficiency of this enzyme leads to an increase in the Ab level in the brain. Neprilysin activity in the brain is declined with age and such a decline has also been observed in subjects just prior to developing Alzheimer’s disease. Therefore, research searching for ways to enhance the function of this enzyme is attracting attention because of its potential for preventing or treating Alzheimer’s disease.
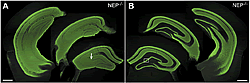
Neprilysin expressed in neurons at the vector-injected side is transported via intrahippocampal neural circuits and localized in the contralateral hippocampus as well as in the injection side.
(A) Hippocampal formations of the vector-injected side. The arrow indicates the site of injection.
(B) The contralateral hippocampal formation to the injection side.
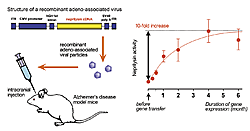
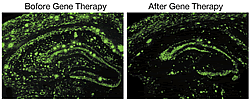
We carried out an experimental gene therapy for Alzheimer’s disease to enhance neprilysin activity in brain and reduce the level of Ab. Introducing neprilysin gene into the brain using an adeno-associated viral vector increase neprilysin activity 10 fold. This increase persisted more than half a year (Fig. 1). Ab levels are increased in the neprilysin-knockout mouse brain, however, this increase was prevented when neprilysin was exogenously introduced. Furthermore, neprilysin expressed in the hippocampal neurons by a single injection at the recombinant viral vector is axonally transported to presynaptic sites through the afferent projections of the neuronal circuits in the contralateral hippocampus (Fig. 2). The Ab level in the contralateral hippocampus was also significantly reduced, just like the vector-injected ipsilateral side, thus showing that neprilysin can degrade Ab at the presynaptic sites. This indicates that increased neprilysin activity can protect synaptic function from those disorders induced by local increases in Ab. We also introduced the gene into a mouse model of Alzheimer’s disease (amyloid precursor protein transgenic mice) and confirmed that the pathological deposition of Ab is significantly reduced when compared to intact model mice and mice injected the viral vector carrying inactivated form of neprilysin gene (Fig. 3).
Neprilysin gene transfer using adeno-associated viral vector has several advantages, such as the non-pathogenic property of the virus, the long-term gene expression, and the restricted localization of gene expression sites. In addition, no noticeable irregularities were observed in mice receiving the neprilysin gene transfer, despite our expectation of side effects. This method is theoretically applicable to humans and we believe it is a promising therapeutic option at a time when no radical therapy is available for Alzheimer’s disease. This gene therapy could potentially become one of the most radical treatments for Alzheimer’s disease patients, including early-onset patients. In addition, the results of the present experiment represent an in vivo demonstration of the possibility of reducing Ab levels by enhancing neprilysin activity in the brain. If future developments of the present research lead to the development of a pharmacological, drug-based method for controlling the neprilysin activity, a even simpler, safer therapy for Alzheimer’s disease might emerge.
Finally, when neprilysin was expressed in the neurons using the adeno-associated viral vector, it was axonally transported, appropriately projected and localized at the presynaptic sites. This means that the neprilysin can be used as a presynaptic marker. Therefore, this vector system may also be useful to visualize specific neural circuits and to examine the formation, reconstruction and morphology of synapses.